Effect of a High-Fat Meal on the Pharmacokinetics of the HIV Integrase Inhibitor Cabotegravir
- PMID: 30230694
- PMCID: PMC6585996
- DOI: 10.1002/cpdd.620
Effect of a High-Fat Meal on the Pharmacokinetics of the HIV Integrase Inhibitor Cabotegravir
Abstract
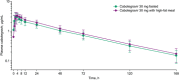
Cabotegravir is an integrase inhibitor in clinical development for the treatment and prevention of HIV infection using oral tablets for short-term, lead-in use before subsequent administration of a long-acting injectable formulation. This phase 1, single-center, randomized, 2 × 2 crossover study evaluated the effect of a high-fat meal on the pharmacokinetics (PK) of oral cabotegravir. Healthy adults received oral cabotegravir 30 mg as a single dose on 2 separate occasions, either after fasting or following a high-fat meal (∼53% fat, ∼870 kcal). Safety evaluations and serial PK samples were collected, and a mixed-effects model was used to determine within-participant treatment comparison of noncompartmental PK parameters. Twenty-four patients were enrolled and had a mean body mass index of 25.6 kg/m2 ; 67% were male. Compared with the fasting state, coadministration of cabotegravir with a high-fat meal increased plasma cabotegravir area under the concentration-time curve and maximal drug concentration, each by 14%. The slight 14% to 17% increase in exposure associated with a high-fat, high-calorie meal was not considered clinically significant. No grade 3/4 adverse events (AEs), drug-related AEs, or AEs leading to discontinuation were reported.
Trial registration: ClinicalTrials.gov NCT02799264.
Keywords: absorption; food; high-fat; integrase inhibitor; pharmacokinetics.
© 2018, The Authors. Clinical Pharmacology in Drug Development published by Wiley Periodicals, Inc. on behalf of The American College of Clinical Pharmacology.
Figures
References
-
- Panel on Antiretroviral Guidelines for Adults and Adolescents . Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents Living with HIV. Rockville, MD: US Department of Health and Human Services; Revised May 30, 2018. http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. Accessed August 6, 2018.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical