Systematic development of temperature shift strategies for Chinese hamster ovary cells based on short duration cultures and kinetic modeling
- PMID: 30230966
- PMCID: PMC6343780
- DOI: 10.1080/19420862.2018.1525262
Systematic development of temperature shift strategies for Chinese hamster ovary cells based on short duration cultures and kinetic modeling
Abstract
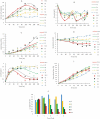
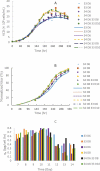
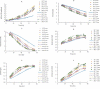
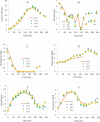
Temperature shift (TS) to a hypothermic condition has been widely used during protein production processes that use Chinese hamster ovary (CHO) cells. The effect of temperature on cell growth, metabolites, protein titer and quality depends on cell line, product, and other bioreactor conditions. Due to the large numbers of experiments, which typically last 2-3 weeks each, limited systematic TS studies have been reported with multiple shift temperatures and steps at different times. Here, we systematically studied the effect of temperature on cell culture performance for the production of two monoclonal antibodies by industrial GS and DG44 CHO cell lines. Three 2-8 day short-duration methods were developed and validated for researching the effect of many different temperatures on CHO cell culture and quality attributes. We found that minor temperature differences (1-1.5 °C) affected cell culture performance. The kinetic parameters extracted from the short duration data were subsequently used to compute and predict cell culture performance in extended duration of 10-14 days with multiple TS conditions for both CHO cell lines. These short-duration culture methods with kinetic modeling tools may be used for effective TS optimization to achieve the best profiles for cell growth, metabolites, titer and quality attributes. Although only three short-duration methods were developed with two CHO cell lines, similar short-duration methods with kinetic modeling may be applied for different hosts, including both microbial and other mammalian cells.
Keywords: CHO cell culture; kinetic modeling; process development; quality attributes; short duration method; specific productivity; temperature shift; titer.
Figures

Similar articles
-
Effective temperature shift strategy development and scale confirmation for simultaneous optimization of protein productivity and quality in Chinese hamster ovary cells.Biotechnol Prog. 2020 May;36(3):e2959. doi: 10.1002/btpr.2959. Epub 2020 Jan 17. Biotechnol Prog. 2020. PMID: 31930722
-
Impacts on product quality attributes of monoclonal antibodies produced in CHO cell bioreactor cultures during intentional mycoplasma contamination events.Biotechnol Bioeng. 2020 Sep;117(9):2802-2815. doi: 10.1002/bit.27436. Epub 2020 Jun 4. Biotechnol Bioeng. 2020. PMID: 32436993 Free PMC article.
-
Kinetic modeling: A tool for temperature shift and feeding optimization in cell culture process development.Protein Expr Purif. 2022 Oct;198:106130. doi: 10.1016/j.pep.2022.106130. Epub 2022 Jun 9. Protein Expr Purif. 2022. PMID: 35691496
-
Factors affecting the quality of therapeutic proteins in recombinant Chinese hamster ovary cell culture.Biotechnol Adv. 2022 Jan-Feb;54:107831. doi: 10.1016/j.biotechadv.2021.107831. Epub 2021 Sep 2. Biotechnol Adv. 2022. PMID: 34480988 Review.
-
A Review on the Current Methods of Chinese Hamster Ovary (CHO) Cells Cultivation for the Production of Therapeutic Protein.Curr Drug Discov Technol. 2021;18(3):354-364. doi: 10.2174/1570163817666200312102137. Curr Drug Discov Technol. 2021. PMID: 32164511 Review.
Cited by
-
Construction of a novel kinetic model for the production process of a CVA6 VLP vaccine in CHO cells.Cytotechnology. 2024 Feb;76(1):69-83. doi: 10.1007/s10616-023-00598-8. Epub 2023 Oct 27. Cytotechnology. 2024. PMID: 38304624 Free PMC article.
-
Process intensification in fed-batch production bioreactors using non-perfusion seed cultures.MAbs. 2019 Nov-Dec;11(8):1502-1514. doi: 10.1080/19420862.2019.1652075. Epub 2019 Aug 19. MAbs. 2019. PMID: 31379298 Free PMC article.
-
Enhancing productivity of Chinese hamster ovary (CHO) cells: synergistic strategies combining low-temperature culture and mTORC1 signaling engineering.Front Bioeng Biotechnol. 2023 Nov 21;11:1268048. doi: 10.3389/fbioe.2023.1268048. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 38076428 Free PMC article.
-
Wheels turning: CHO cell modeling moves into a digital biomanufacturing era: Subtitle: CHO Metabolic Modeling.Comput Struct Biotechnol J. 2025 Jun 23;27:2796-2813. doi: 10.1016/j.csbj.2025.06.035. eCollection 2025. Comput Struct Biotechnol J. 2025. PMID: 40677243 Free PMC article. Review.
-
Temperature Upshifts in Mammalian Cell Culture: A Suitable Strategy for Biosimilar Monoclonal Antibodies?Bioengineering (Basel). 2023 Sep 30;10(10):1149. doi: 10.3390/bioengineering10101149. Bioengineering (Basel). 2023. PMID: 37892879 Free PMC article.
References
-
- Wurm FM. CHO Quasispecies—implications for Manufacturing Processes. Processes. 2013;1:296–311. doi:10.3390/pr1030296. - DOI
-
- Xu J, Jin M, Song H, Huang C, Xu X, Tian J, Qian N-X, Steger K, Lewen NS, Tao L, et al. Brown drug substance color investigation in cell culture manufacturing using chemically defined media: A case study. Process Biochem. 2014;49:130–139. doi:10.1016/j.procbio.2013.10.015. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
