Therapeutic potential of extracellular vesicles derived from human mesenchymal stem cells in a model of progressive multiple sclerosis
- PMID: 30231069
- PMCID: PMC6145506
- DOI: 10.1371/journal.pone.0202590
Therapeutic potential of extracellular vesicles derived from human mesenchymal stem cells in a model of progressive multiple sclerosis
Abstract
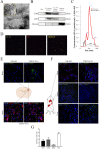
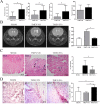
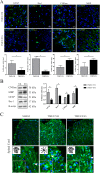
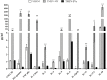
Extracellular vesicles (EVs) have emerged as important mediators of intercellular communication and as possible therapeutic agents in inflammation-mediated demyelinating diseases, including multiple sclerosis (MS). In the present study, we investigated whether intravenously administered EVs derived from mesenchymal stem cells (MSCs) from human adipose tissue might mediate recovery in Theiler's murine encephalomyelitis virus (TMEV)-induced demyelinating disease, a progressive model of MS. SJL/J mice were subjected to EV treatment once the disease was established. We found that intravenous EV administration improved motor deficits, reduced brain atrophy, increased cell proliferation in the subventricular zone and decreased inflammatory infiltrates in the spinal cord in mice infected with TMEV. EV treatment was also capable of modulating neuroinflammation, given glial fibrillary acidic protein and Iba-1 staining were reduced in the brain, whereas myelin protein expression was increased. Changes in the morphology of microglial cells in the spinal cord suggest that EVs also modulate the activation state of microglia. The clear reduction in plasma cytokine levels, mainly in the Th1 and Th17 phenotypes, in TMEV mice treated with EVs confirms the immunomodulatory ability of intravenous EVs. According to our results, EV administration attenuates motor deficits through immunomodulatory actions, diminishing brain atrophy and promoting remyelination. Further studies are necessary to establish EV delivery as a possible therapy for the neurodegenerative phase of MS.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Comment in
-
Editorial: Innate Immunity Pathways in Autoimmune Diseases.Front Immunol. 2019 Jun 4;10:1245. doi: 10.3389/fimmu.2019.01245. eCollection 2019. Front Immunol. 2019. PMID: 31214194 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

