Transglutaminase 2 Promotes Autophagy by LC3 Induction through p53 Depletion in Cancer Cell
- PMID: 30231606
- PMCID: PMC6319544
- DOI: 10.4062/biomolther.2018.140
Transglutaminase 2 Promotes Autophagy by LC3 Induction through p53 Depletion in Cancer Cell
Abstract
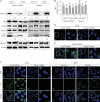
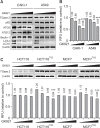
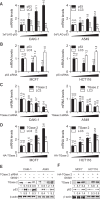
Transglutaminase 2 (TGase 2) plays a key role in p53 regulation, depleting p53 tumor suppressor through autophagy in renal cell carcinoma. We found that microtubule-associated protein 1A/1B-light chain 3 (LC3), a hallmark of autophagy, were tightly associated with the level of TGase 2 in cancer cells. TGase 2 overexpression increased LC3 levels, and TGase 2 knockdown decreased LC3 levels in cancer cells. Transcript abundance of LC3 was inversely correlated with level of wild type p53. TGase 2 knockdown using siRNA, or TGase 2 inhibition using GK921 significantly reduced autophagy through reduction of LC3 transcription, which was followed by restoration of p53 levels in cancer cells. TGase 2 overexpression promoted the autophagy process by LC3 induction, which was correlated with p53 depletion in cancer cells. Rapamycin-resistant cancer cells also showed higher expression of LC3 compared to the rapamycin-sensitive cancer cells, which was tightly correlated with TGase 2 levels. TGase 2 knockdown or TGase 2 inhibition sensitized rapamycin-resistant cancer cells to drug treatment. In summary, TGase 2 induces drug resistance by potentiating autophagy through LC3 induction via p53 regulation in cancer.
Keywords: Autophagy; Cancer cell; LC3; Transglutaminase 2; p53.
Figures

References
-
- Birgisdottir AB, Lamark T, Johansen T. The LIR motif - crucial for selective autophagy. J. Cell Sci. 2013;126:3237–3247. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

