Sensory Processing Phenotypes in Fragile X Syndrome
- PMID: 30231625
- PMCID: PMC6149018
- DOI: 10.1177/1759091418801092
Sensory Processing Phenotypes in Fragile X Syndrome
Abstract
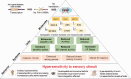
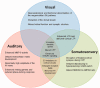
Fragile X syndrome (FXS) is a neurodevelopmental disorder that causes intellectual disability. It is a leading known genetic cause of autism. In addition to cognitive, social, and communication deficits, humans with FXS demonstrate abnormal sensory processing including sensory hypersensitivity. Sensory hypersensitivity commonly manifests as auditory, tactile, or visual defensiveness or avoidance. Clinical, behavioral, and electrophysiological studies consistently show auditory hypersensitivity, impaired habituation to repeated sounds, and reduced auditory attention in humans with FXS. Children with FXS also exhibit significant visuospatial impairments. Studies in infants and toddlers with FXS have documented impairments in processing texture-defined motion stimuli, temporal flicker, perceiving ordinal numerical sequence, and the ability to maintain the identity of dynamic object information during occlusion. Consistent with the observations in humans with FXS, fragile X mental retardation 1 ( Fmr1) gene knockout (KO) rodent models of FXS also show seizures, abnormal visual-evoked responses, auditory hypersensitivity, and abnormal processing at multiple levels of the auditory system, including altered acoustic startle responses. Among other sensory symptoms, individuals with FXS exhibit tactile defensiveness. Fmr1 KO mice also show impaired encoding of tactile stimulation frequency and larger size of receptive fields in the somatosensory cortex. Since sensory deficits are relatively more tractable from circuit mechanisms and developmental perspectives than more complex social behaviors, the focus of this review is on clinical, functional, and structural studies that outline the auditory, visual, and somatosensory processing deficits in FXS. The similarities in sensory phenotypes between humans with FXS and animal models suggest a likely conservation of basic sensory processing circuits across species and may provide a translational platform to not just develop biomarkers but also to understand underlying mechanisms. We argue that preclinical studies in animal models of FXS can facilitate the ongoing search for new therapeutic approaches in FXS by understanding mechanisms of basic sensory processing circuits and behaviors that are conserved across species.
Keywords: autism spectrum disorders; fragile X syndrome; neurodevelopmental disorders; sensory hypersensitivity.
Figures
References
-
- Abe M., Furukawa S., Takayama S., Michitaka K., Minami H., Yamamoto K., Horiike N., Onji M. (2003). Drug-induced hepatitis with autoimmune features during minocycline therapy. Intern Med, 42, 48–52. - PubMed
-
- Adusei D. C., Pacey L. K., Chen D., Hampson D. R. (2010). Early developmental alterations in GABAergic protein expression in fragile X knockout mice. Neuropharmacology, 59, 167–171. - PubMed
-
- Akin E., Miller L. C., Tucker L. B. (1998). Minocycline-induced lupus in adolescents. Pediatrics, 101, 926. - PubMed
-
- Allen C. B., Celikel T., Feldman D. E. (2003). Long-term depression induced by sensory deprivation during cortical map plasticity in vivo. Nat Neurosci, 6, 291–299. - PubMed
-
- Ang E. R., Zimmerman J. C., Malkin E. (2002). Pseudotumor cerebri secondary to minocycline intake. J Am Board Fam Pract, 15, 229–233. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
