Transcriptome dynamics of rooting zone and aboveground parts of cuttings during adventitious root formation in Cryptomeria japonica D. Don
- PMID: 30231856
- PMCID: PMC6148763
- DOI: 10.1186/s12870-018-1401-7
Transcriptome dynamics of rooting zone and aboveground parts of cuttings during adventitious root formation in Cryptomeria japonica D. Don
Abstract
Background: Adventitious root formation is an essential physiological process for successful propagation of cuttings in various plant species. Because coniferous species are highly heterozygous, propagation of cuttings is of great practical use in breeding. Although various factors influence adventitious root formation, little is known of the associated regulatory mechanisms. Whereas adventitious roots generally form from the base of cuttings, this process is accompanied by physiological changes in leaves, which supply assimilates and metabolites. Herein, we present microarray analyses of transcriptome dynamics during adventitious root formation in whole cuttings in the coniferous species, Cryptomeria japonica.
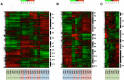
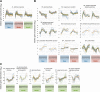
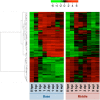
Results: Temporal patterns of gene expression were determined in the base, the middle, and needles of cuttings at eight time points during adventitious root formation. Global gene expression at the base had diverged from that in the middle by 3-h post-insertion, and changed little in the subsequent 3-days post-insertion, and global gene expression in needles altered characteristically at 3- and 6-weeks post-insertion. In Gene Ontology enrichment analysis of major gene clusters based on hierarchical clustering, the expression profiles of genes related to carbohydrates, plant hormones, and other categories indicated multiple biological changes that were involved in adventitious root formation.
Conclusions: The present comprehensive transcriptome analyses indicate major transcriptional turning and contribute to the understanding of the biological processes and molecular factors that influence adventitious root formation in C. japonica.
Keywords: Adventitious root formation; Conifer; Cryptomeria japonica; Microarray; Needles; Transcriptome.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures






Similar articles
-
Transcriptomic profiling and discovery of key genes involved in adventitious root formation from green cuttings of highbush blueberry (Vaccinium corymbosum L.).BMC Plant Biol. 2020 Apr 25;20(1):182. doi: 10.1186/s12870-020-02398-0. BMC Plant Biol. 2020. PMID: 32334538 Free PMC article.
-
Localized gene expression changes during adventitious root formation in black walnut (Juglans nigra L.).Tree Physiol. 2018 Jun 1;38(6):877-894. doi: 10.1093/treephys/tpx175. Tree Physiol. 2018. PMID: 29378021
-
Gene expression profiling during adventitious root formation in carnation stem cuttings.BMC Genomics. 2015 Oct 14;16:789. doi: 10.1186/s12864-015-2003-5. BMC Genomics. 2015. PMID: 26467528 Free PMC article.
-
Adventitious root formation in tree species: involvement of transcription factors.Physiol Plant. 2014 Jun;151(2):192-8. doi: 10.1111/ppl.12197. Physiol Plant. 2014. PMID: 24666319 Review.
-
Auxin-Induced Adventitious Root Formation in Nodal Cuttings of Camellia sinensis.Int J Mol Sci. 2019 Sep 27;20(19):4817. doi: 10.3390/ijms20194817. Int J Mol Sci. 2019. PMID: 31569758 Free PMC article. Review.
Cited by
-
A protocol for Agrobacterium-mediated transformation of Japanese cedar, Sugi (Cryptomeria japonica D. Don) using embryogenic tissue explants.Plant Biotechnol (Tokyo). 2020 Jun 25;37(2):147-156. doi: 10.5511/plantbiotechnology.20.0131a. Plant Biotechnol (Tokyo). 2020. PMID: 32821221 Free PMC article.
-
Transcriptomic profiling and discovery of key genes involved in adventitious root formation from green cuttings of highbush blueberry (Vaccinium corymbosum L.).BMC Plant Biol. 2020 Apr 25;20(1):182. doi: 10.1186/s12870-020-02398-0. BMC Plant Biol. 2020. PMID: 32334538 Free PMC article.
-
Advanced technologies for reducing greenhouse gas emissions from rice fields: Is hybrid rice the game changer?Plant Commun. 2025 Feb 10;6(2):101224. doi: 10.1016/j.xplc.2024.101224. Epub 2024 Dec 28. Plant Commun. 2025. PMID: 39936846 Free PMC article. Review.
-
A Perspective on Adventitious Root Formation in Tree Species.Plants (Basel). 2020 Dec 17;9(12):1789. doi: 10.3390/plants9121789. Plants (Basel). 2020. PMID: 33348577 Free PMC article.
-
Adventitious Root Formation in Tree Species.Plants (Basel). 2021 Mar 5;10(3):486. doi: 10.3390/plants10030486. Plants (Basel). 2021. PMID: 33807512 Free PMC article.
References
-
- de Klerk GJ, van der Krieken W, de Jong JC. Review the formation of adventitious roots: new concepts, new possibilities. In Vitro Cell Dev Biol Plant. 1999;35(3):189–199. doi: 10.1007/s11627-999-0076-z. - DOI
-
- Pop TI, Pamfil D, Bellini C. Auxin control in the formation of adventitious roots. Not Bot Hort Agrobot Cluj. 2011;39(1):307–316. doi: 10.15835/nbha3916101. - DOI
-
- Gutierrez L, Mongelard G, Floková K, Păcurar DI, Novák O, Staswick P, Kowalczyk M, Păcurar M, Demailly H, Geiss G, Bellini C. Auxin controls Arabidopsis adventitious root initiation by regulating jasmonic acid homeostasis. Plant Cell. 2012;24(6):2515–2527. doi: 10.1105/tpc.112.099119. - DOI - PMC - PubMed
-
- Ahkami AH, Melzer M, Ghaffari MR, Pollmann S, Javid MG, Shahinnia F, Hajirezaei MR, Druege U. Distribution of indole-3-acetic acid in Petunia hybrida shoot tip cuttings and relationship between auxin transport, carbohydrate metabolism and adventitious root formation. Planta. 2013;238(3):499–517. doi: 10.1007/s00425-013-1907-z. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

