Amyloid positron emission tomography and cerebrospinal fluid results from a crenezumab anti-amyloid-beta antibody double-blind, placebo-controlled, randomized phase II study in mild-to-moderate Alzheimer's disease (BLAZE)
- PMID: 30231896
- PMCID: PMC6146627
- DOI: 10.1186/s13195-018-0424-5
Amyloid positron emission tomography and cerebrospinal fluid results from a crenezumab anti-amyloid-beta antibody double-blind, placebo-controlled, randomized phase II study in mild-to-moderate Alzheimer's disease (BLAZE)
Abstract
Background: We investigated the effect of crenezumab, a humanized anti-amyloid-beta (Aβ) immunoglobulin (Ig)G4 monoclonal antibody, on biomarkers of amyloid pathology, neurodegeneration, and disease progression in patients with mild-to-moderate Alzheimer's disease (AD).
Methods: This double-blind, placebo-controlled, randomized phase II study enrolled patients with mild-to-moderate AD and a Mini-Mental State Examination (MMSE) score of 18-26. In part 1 of the study, patients were 2:1 randomized to receive low-dose subcutaneous (SC) 300 mg crenezumab every 2 weeks (q2w) or placebo for 68 weeks; in part 2, patients were 2:1 randomized to receive high-dose intravenous (IV) 15 mg/kg crenezumab every 4 weeks (q4w) or placebo for 68 weeks. The primary endpoint was change in amyloid burden from baseline to week 69 assessed by florbetapir positron emission tomography (PET) in the modified intent-to-treat population. Secondary endpoints were change from baseline to week 69 in cerebrospinal fluid (CSF) biomarkers and fluorodeoxyglucose PET, and change from baseline to week 73 in 12-point Alzheimer's Disease Assessment Scale cognitive subscale (ADAS-Cog12) and Clinical Dementia Rating Sum of Boxes (CDR-SB). Safety was assessed in patients who received at least one dose of study treatment.
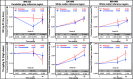
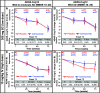
Results: From August 2011 to September 2012, 91 patients were enrolled and randomized (low-dose SC cohort: crenezumab (n = 26) or placebo (n = 13); high-dose IV cohort: crenezumab (n = 36) or placebo (n = 16)). The primary endpoint was not met using a prespecified cerebellar reference region to calculate standard uptake value ratios (SUVRs) from florbetapir PET. Exploratory analyses using subcortical white matter reference regions showed nonsignificant trends toward slower accumulation of plaque amyloid in the high-dose IV cohort. In both cohorts, a significant mean increase from baseline in CSF Aβ(1-42) levels versus placebo was observed. Nonsignificant trends toward ADAS-Cog12 and CDR-SB benefits were identified in a mild (MMSE 20-26) subset of the high-dose IV cohort. No amyloid-related imaging abnormalities due to edema/effusion were observed.
Conclusion: The primary endpoint was not met. Exploratory findings suggest potential Aβ target engagement with crenezumab and possible slower accumulation of plaque amyloid. Studies investigating the effects of higher doses of crenezumab on amyloid load and disease progression are ongoing.
Trial registration: ClinicalTrials.gov, NCT01397578 . Registered on 18 July 2011.
Keywords: Alzheimer’s disease; Antibodies; Biomarkers; Humanized; Monoclonal antibodies; Positron emission tomography.
Conflict of interest statement
Authors’ information
MW, CH, SSu, and RP were employees of Genentech Inc., South San Francisco, CA, USA at the time of the study.
Ethics approval and consent to participate
This study was conducted at 21 sites in the US, one site in Spain, and one site in France. The study protocol was approved by the respective institutional review boards prior to participant recruitment and was conducted in accordance with US Food and Drug Administration regulations, International Council on Harmonization E6 Guideline for Good Clinical Practice, and applicable local, state, federal, and country laws. Written informed consent was obtained for all patients prior to performing study-related procedures in accordance with federal and institutional guidelines.
Consent for publication
Not applicable.
Competing interests
LAH, WC, MW, MF, FB, AQ, DC, DM, TB, CH, CR, SPS, KRW, SSu, RNF, and RP are current or former employees of Genentech (a member of the Roche Group), and own stock or stock options in F. Hoffmann-La Roche. SSa received grants and personal fees from Genentech during the conduct of the study, grants and personal fees from Eli Lilly, Biogen Idec, Merck, and Roche, and grants from Functional Neuromodulation, Avid, and Novartis. EMR received grants from Banner Alzheimer’s Institute during the conduct of the study, and is evaluating crenezumab in the Alzheimer’s Prevention Initiative (API) Autosomal Dominant Alzheimer’s Disease Trial. KC declares that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures



References
-
- Adolfsson O, Pihlgren M, Toni N, Varisco Y, Buccarello AL, Antoniello K, et al. An effector-reduced anti-β-amyloid (Aβ) antibody with unique Aβ binding properties promotes neuroprotection and glial engulfment of Aβ. J Neurosci. 2012;32:9677–9689. doi: 10.1523/JNEUROSCI.4742-11.2012. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

