Deletion of the microtubule-associated protein 6 (MAP6) results in skeletal muscle dysfunction
- PMID: 30231928
- PMCID: PMC6147105
- DOI: 10.1186/s13395-018-0176-8
Deletion of the microtubule-associated protein 6 (MAP6) results in skeletal muscle dysfunction
Abstract
Background: The skeletal muscle fiber has a specific and precise intracellular organization which is at the basis of an efficient muscle contraction. Microtubules are long known to play a major role in the function and organization of many cells, but in skeletal muscle, the contribution of the microtubule cytoskeleton to the efficiency of contraction has only recently been studied. The microtubule network is dynamic and is regulated by many microtubule-associated proteins (MAPs). In the present study, the role of the MAP6 protein in skeletal muscle organization and function has been studied using the MAP6 knockout mouse line.
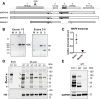
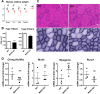
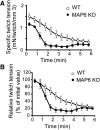
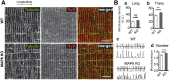
Methods: The presence of MAP6 transcripts and proteins was shown in mouse muscle homogenates and primary culture using RT-PCR and western blot. The in vivo evaluation of muscle force of MAP6 knockout (KO) mice was performed on anesthetized animals using electrostimulation coupled to mechanical measurement and multimodal magnetic resonance. The impact of MAP6 deletion on microtubule organization and intracellular structures was studied using immunofluorescent labeling and electron microscopy, and on calcium release for muscle contraction using Fluo-4 calcium imaging on cultured myotubes. Statistical analysis was performed using Student's t test or the Mann-Whitney test.
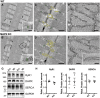
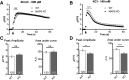
Results: We demonstrate the presence of MAP6 transcripts and proteins in skeletal muscle. Deletion of MAP6 results in a large number of muscle modifications: muscle weakness associated with slight muscle atrophy, alterations of microtubule network and sarcoplasmic reticulum organization, and reduction in calcium release.
Conclusion: Altogether, our results demonstrate that MAP6 is involved in skeletal muscle function. Its deletion results in alterations in skeletal muscle contraction which contribute to the global deleterious phenotype of the MAP6 KO mice. As MAP6 KO mouse line is a model for schizophrenia, our work points to a possible muscle weakness associated to some forms of schizophrenia.
Keywords: Calcium release; Microtubule-associated protein; Microtubules; Muscle contraction; Sarcoplasmic reticulum; Triad.
Conflict of interest statement
Ethics approval
All procedures using animals were approved by the institutional ethics committee (
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Khairallah RJ, Shi G, Sbrana F, Prosser BL, Borroto C, Mazaitis MJ, Hoffman EP, Mahurkar A, Sachs F, Sun Y, Chen YW, Raiteri R, Lederer WJ, Dorsey SG, Ward CW. Microtubules underlie dysfunction in duchenne muscular dystrophy. Sci Signal. 2012;5:ra56. doi: 10.1126/scisignal.2002829. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

