SYK-CARD9 Signaling Axis Promotes Gut Fungi-Mediated Inflammasome Activation to Restrict Colitis and Colon Cancer
- PMID: 30231985
- PMCID: PMC6541497
- DOI: 10.1016/j.immuni.2018.08.024
SYK-CARD9 Signaling Axis Promotes Gut Fungi-Mediated Inflammasome Activation to Restrict Colitis and Colon Cancer
Abstract
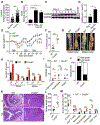
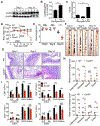
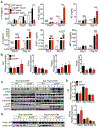
Fungi represent a significant proportion of the gut microbiota. Aberrant immune responses to fungi are frequently observed in inflammatory bowel diseases (IBD) and colorectal cancer (CRC), and mutations in the fungal-sensing pathways are associated with the pathogenesis of IBD. Fungal recognition receptors trigger downstream signaling via the common adaptor protein CARD9 and the kinase SYK. Here we found that commensal gut fungi promoted inflammasome activation during AOM-DSS-induced colitis. Myeloid cell-specific deletion of Card9 or Syk reduced inflammasome activation and interleukin (IL)-18 maturation and increased susceptibility to colitis and CRC. IL-18 promoted epithelial barrier restitution and interferon-γ production by intestinal CD8+ T cells. Supplementation of IL-18 or transfer of wild-type myeloid cells reduced tumor burden in AOM-DSS-treated Card9-/- and Sykfl/flLysMCre/+ mice, whereas treatment with anti-fungal agents exacerbated colitis and CRC. CARD9 deletion changes the gut microbial landscape, suggesting that SYK-CARD9 signaling maintains a microbial ecology that promotes inflammasome activation and thereby restrains colitis and colon tumorigenesis.
Keywords: CARD9; SYK; colorectal cancer; inflammatory bowel diseases; microbiota.
Copyright © 2018 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests
The authors declare no competing interests.
Figures




Comment in
-
Fungi Enter the Stage of Colon Carcinogenesis.Immunity. 2018 Sep 18;49(3):384-386. doi: 10.1016/j.immuni.2018.09.002. Immunity. 2018. PMID: 30231977
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

