Improved methods for MRI-compatible implants in nonhuman primates
- PMID: 30232039
- PMCID: PMC6203806
- DOI: 10.1016/j.jneumeth.2018.09.013
Improved methods for MRI-compatible implants in nonhuman primates
Abstract
Background: Neuroscientists commonly use permanently implanted headposts to stabilize the head of nonhuman primates (NHPs) during electrophysiology and functional magnetic resonance imaging (fMRI). Here, we present improved methodology for MRI-compatible implants without the use of acrylic for head stabilization in NHPs.
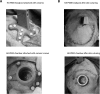
New method: MRI is used to obtain a 3D-reconstruction of NHP skulls, which are used to create customized implants by modeling intersections with the bone. Implants are manufactured from PEEK using computer numerical control machining and coated with hydroxyapatite to promote osseointegration. Surgically, implants are attached to the skull with ceramic screws, while the skin flap is pulled over the implant and closed subcutaneously.
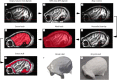
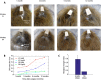
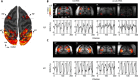
Results: Quality of blood oxygen level dependent (BOLD) fMRI signal is improved in animals implanted with our method as compared to traditional acrylic implants. Additionally, implants are well-integrated with the skull, remain robust for more than a year and without granulation tissue around the skin margin.
Comparison with existing method(s): Previous improvements on NHP implants (Chen et al., 2017; McAndrew et al., 2012; Mulliken et al., 2015; Overton et al., 2017) lacked fMRI-compatibility, as they relied on titanium headposts and/or titanium screws. Thus, most fMRI studies in NHPs today still rely on the use of acrylic-based headposts for stabilization and the use of contrast-enhanced agents to improve MRI signal.
Conclusions: Our method preserves fMRI-compatibility and results in measurable improvement in BOLD signal without the use of contrast-enhanced agents. Furthermore, the long-term stability of our implants contributes positively to the wellbeing of NHPs in neuroscience research.
Keywords: Acrylic; Electrophysiology; Functional magnetic resonance imaging (fMRI); Hydroxyapatite (HA); Implant; Macaque; Osseointegration; Polyetheretherketone (PEEK).
Copyright © 2018 The Author(s). Published by Elsevier B.V. All rights reserved.
Figures
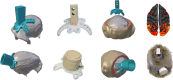




References
-
- Cox R.W. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. 1996;29:162–173. - PubMed
-
- Goense J.B.M., Ku S.P., Merkle H., Tolias A.S., Logothetis N.K. fMRI of the temporal lobe of the awake monkey at 7 T. Neuroimage. 2008;39:1081–1093. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

