Emerging mechanisms of asymmetric stem cell division
- PMID: 30232100
- PMCID: PMC6219723
- DOI: 10.1083/jcb.201807037
Emerging mechanisms of asymmetric stem cell division
Abstract
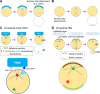
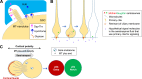
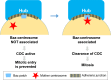
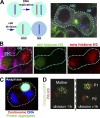
The asymmetric cell division of stem cells, which produces one stem cell and one differentiating cell, has emerged as a mechanism to balance stem cell self-renewal and differentiation. Elaborate cellular mechanisms that orchestrate the processes required for asymmetric cell divisions are often shared between stem cells and other asymmetrically dividing cells. During asymmetric cell division, cells must establish asymmetry/polarity, which is guided by varying degrees of intrinsic versus extrinsic cues, and use intracellular machineries to divide in a desired orientation in the context of the asymmetry/polarity. Recent studies have expanded our knowledge on the mechanisms of asymmetric cell divisions, revealing the previously unappreciated complexity in setting up the cellular and/or environmental asymmetry, ensuring binary outcomes of the fate determination. In this review, we summarize recent progress in understanding the mechanisms and regulations of asymmetric stem cell division.
© 2018 Venkei and Yamashita.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

