Evaluation of Risk Factors for Clostridium difficile Infection Based on Immunochromatography Testing and Toxigenic Culture Assay
- PMID: 30232129
- PMCID: PMC6258861
- DOI: 10.1128/JCM.00555-18
Evaluation of Risk Factors for Clostridium difficile Infection Based on Immunochromatography Testing and Toxigenic Culture Assay
Abstract
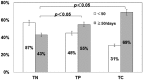
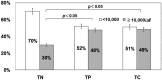
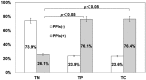
In recent years, the diagnostic method of choice for Clostridium difficile infection (CDI) is a rapid enzyme immunoassay in which glutamate dehydrogenase (GDH) antigen and C. difficile toxin can be detected (C. diff Quik Chek Complete; Alere Inc.) (Quik Chek). However, the clinical significance remains unclear in cases that demonstrate a positive result for GDH antigen and are negative for toxin. In this study, we used the Quik Chek test kit on fecal samples, with an additional toxin detection step using a toxigenic culture assay for the aforementioned cases. CDI risk factors were assessed among the 3 groups divided by the Quik Chek test results. The study involved 1,565 fecal samples from patients suspected to have CDI who were hospitalized during the period of April 2012 to March 2014. The 3 groups were defined as follows: both GDH antigen positive and toxin positive (by Quik Chek test) (toxin-positive [TP] group, n = 109), both GDH antigen and toxin negative (toxin-negative [TN] group, n = 111), and positive only for GDH antigen but toxin positive with subsequent toxigenic culture (toxigenic culture [TC] group, n = 72). The gender, age, number of hospitalization days, white blood cell (WBC) counts, serum albumin levels, body mass index (BMI), fecal consistency, and use of antibacterials and proton pump inhibiters (PPIs) were analyzed. The positive rate for the fecal direct Quik Chek test was 7.0% (109/1,565 cases). However, toxigenic culture assays using the Quik Chek test for only the GDH-antigen-positive/toxin-negative samples were 35.3% positive (72/204 cases). As a result, the true positive rate for C. difficile toxin detection was estimated to be 11.6% (181/1,565 cases). Moreover, significant differences (P < 0.05) in the number of hospitalization days (>50 days), WBC counts (>10,000 WBCs/μl), and use of PPIs comparing the TN, TP, and TC groups, were observed. The odds ratios (ORs) for the development of CDI were 1.61 (95% confidence interval [CI], 0.94 to 2.74) and 2.98 (95% CI, 1.59 to 5.58) for numbers of hospitalization days, 2.16 (95% CI, 1.24 to 3.75) and 2.24 (95% CI, 1.21 to 4.14) for WBC counts, and 9.03 (95% CI, 4.9 to 16.6) and 9.15 (95% CI, 4.59 to 18.2) for use of PPIs in the TP and TC groups, respectively. These findings demonstrated that the use of PPIs was a significant risk factor for CDI development. Moreover, antibacterials such as carbapenems, cephalosporins, and fluoroquinolones were demonstrated to be risk factors. In conclusion, identification of the TC group of patients is thought to be important, as this study demonstrates that this group bears the same high risk of developing CDI as the TP group.
Keywords: C. diff Quik Chek Complete test; Clostridium difficile infection; glutamate dehydrogenase; risk factor; toxigenic culture assay.
Copyright © 2018 American Society for Microbiology.
Figures
References
-
- Kato H, Kita H, Karasawa T, Maegawa T, Koino Y, Takakuwa H, Saikai T, Kobayashi K, Yamagishi T, Nakamura S. 2001. Colonisation and transmission of Clostridium difficile in healthy individuals examined by PCR ribotyping and pulsed-field gel electrophoresis. J Med Microbiol 50:720–727. doi: 10.1099/0022-1317-50-8-720. - DOI - PubMed
-
- Matsuki S, Ozaki E, Shozu M, Inoue M, Shimizu S, Yamaguchi N, Karasawa T, Yamagishi T, Nakamura S. 2005. Colonization by Clostridium difficile of neonates in a hospital, and infants and children in three day-care facilities of Kanazawa, Japan. Int Microbiol 8:43–48. - PubMed
-
- Cohen SH, Gerding DN, Johnson S, Kelly CP, Loo VG, McDonald LC, Pepin J, Wilcox MH. 2010. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol 31:431–455. doi: 10.1086/651706. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

