Histone Deacetylase Inhibitors Synergize with Catalytic Inhibitors of EZH2 to Exhibit Antitumor Activity in Small Cell Carcinoma of the Ovary, Hypercalcemic Type
- PMID: 30232145
- PMCID: PMC6279577
- DOI: 10.1158/1535-7163.MCT-18-0348
Histone Deacetylase Inhibitors Synergize with Catalytic Inhibitors of EZH2 to Exhibit Antitumor Activity in Small Cell Carcinoma of the Ovary, Hypercalcemic Type
Abstract
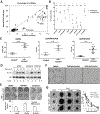
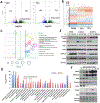
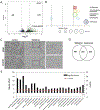
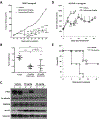
Small cell carcinoma of the ovary, hypercalcemic type (SCCOHT) is a rare but extremely lethal malignancy that mainly impacts young women. SCCOHT is characterized by a diploid genome with loss of SMARCA4 and lack of SMARCA2 expression, two mutually exclusive ATPases of the SWI/SNF chromatin-remodeling complex. We and others have identified the histone methyltransferase EZH2 as a promising therapeutic target for SCCOHT, suggesting that SCCOHT cells depend on the alternation of epigenetic pathways for survival. In this study, we found that SCCOHT cells were more sensitive to pan-HDAC inhibitors compared with other ovarian cancer lines or immortalized cell lines tested. Pan-HDAC inhibitors, such as quisinostat, reversed the expression of a group of proteins that were deregulated in SCCOHT cells due to SMARCA4 loss, leading to growth arrest, apoptosis, and differentiation in vitro and suppressed tumor growth of xenografted tumors of SCCOHT cells. Moreover, combined treatment of HDAC inhibitors and EZH2 inhibitors at sublethal doses synergistically induced histone H3K27 acetylation and target gene expression, leading to rapid induction of apoptosis and growth suppression of SCCOHT cells and xenografted tumors. Therefore, our preclinical study highlighted the therapeutic potential of combined treatment of HDAC inhibitors with EZH2 catalytic inhibitors to treat SCCOHT.
©2018 American Association for Cancer Research.
Conflict of interest statement
Figures


References
-
- Florell SR, Bruggers CS, Matlak M, Young RH, Lowichik A. Ovarian small cell carcinoma of the hypercalcemic type in a 14 month old: the youngest reported case. Med Pediatr Oncol 1999;32(4):304–7. - PubMed
-
- Young RH, Oliva E, Scully RE. Small cell carcinoma of the ovary, hypercalcemic type. A clinicopathological analysis of 150 cases. Am J Surg Pathol 1994;18(11):1102–16. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

