Attenuation of Influenza A Virus Disease Severity by Viral Coinfection in a Mouse Model
- PMID: 30232180
- PMCID: PMC6232468
- DOI: 10.1128/JVI.00881-18
Attenuation of Influenza A Virus Disease Severity by Viral Coinfection in a Mouse Model
Abstract
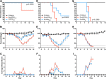
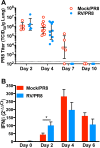
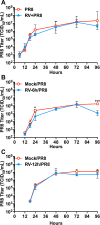
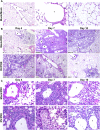
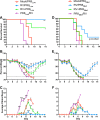
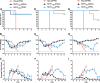
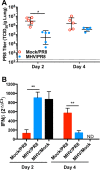
Influenza viruses and rhinoviruses are responsible for a large number of acute respiratory viral infections in human populations and are detected as copathogens within hosts. Clinical and epidemiological studies suggest that coinfection by rhinovirus and influenza virus may reduce disease severity and that they may also interfere with each other's spread within a host population. To determine how coinfection by these two unrelated respiratory viruses affects pathogenesis, we established a mouse model using a minor serogroup rhinovirus (rhinovirus strain 1B [RV1B]) and mouse-adapted influenza A virus (A/Puerto Rico/8/1934 [PR8]). Infection of mice with RV1B 2 days before PR8 reduced the severity of infection by a low or medium, but not high, dose of PR8. Disease attenuation was associated with an early inflammatory response in the lungs and enhanced clearance of PR8. However, coinfection by RV1B did not reduce PR8 viral loads early in infection or inhibit replication of PR8 within respiratory epithelia or in vitro Inflammation in coinfected mice remained focal compared to diffuse inflammation and damage in the lungs of mice infected by PR8. The timing of RV1B coinfection was a critical determinant of protection, suggesting that sufficient time is needed to induce this response. Finally, disease attenuation was not unique to RV1B: dose-dependent coinfection by a murine coronavirus (mouse hepatitis virus strain 1 [MHV-1]) also reduced the severity of PR8 infection. Unlike RV1B, coinfection with MHV-1 reduced early PR8 replication, which was associated with upregulation of beta interferon (IFN-β) expression. This model is critical for understanding the mechanisms responsible for influenza disease attenuation during coinfection by unrelated respiratory viruses.IMPORTANCE Viral infections in the respiratory tract can cause severe disease and are responsible for a majority of pediatric hospitalizations. Molecular diagnostics have revealed that approximately 20% of these patients are infected by more than one unrelated viral pathogen. To understand how viral coinfection affects disease severity, we inoculated mice with a mild viral pathogen (rhinovirus or murine coronavirus), followed 2 days later by a virulent viral pathogen (influenza A virus). This model demonstrated that rhinovirus can reduce the severity of influenza A virus, which corresponded with an early but controlled inflammatory response in the lungs and early clearance of influenza A virus. We further determined the dose and timing parameters that were important for effective disease attenuation and showed that influenza disease is also reduced by coinfection with a murine coronavirus. These findings demonstrate that coinfecting viruses can alter immune responses and pathogenesis in the respiratory tract.
Keywords: coinfection; coronavirus; immunopathogenesis; influenza; mouse model; rhinovirus.
Copyright © 2018 American Society for Microbiology.
Figures

References
-
- Nolan VG, Arnold SR, Bramley AM, Ampofo K, Williams DJ, Grijalva CG, Self WH, Anderson EJ, Wunderink RG, Edwards KM, Pavia AT, Jain S, McCullers JA. 2018. Etiology and impact of coinfections in children hospitalized with community-acquired pneumonia. J Infect Dis 218:179–188. doi: 10.1093/infdis/jix641. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

