Cellular Protein Kinase D Modulators Play a Role during Multiple Steps of Herpes Simplex Virus 1 Egress
- PMID: 30232182
- PMCID: PMC6232494
- DOI: 10.1128/JVI.01486-18
Cellular Protein Kinase D Modulators Play a Role during Multiple Steps of Herpes Simplex Virus 1 Egress
Abstract
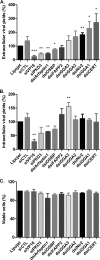
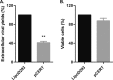
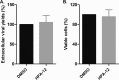
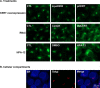
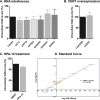
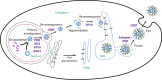
The assembly of new herpes simplex virus 1 (HSV-1) particles takes place in the nucleus. These particles then travel across the two nuclear membranes and acquire a final envelope from a cellular compartment. The contribution of the cell to the release of the virus is, however, little known. We previously demonstrated, using a synchronized infection, that the host protein kinase D and diacylglycerol, a lipid that recruits the kinase to the trans-Golgi network (TGN), promote the release of the virus from that compartment. Given the role this cellular protein plays in the herpes simplex virus 1 life cycle and the many molecules that modulate its activity, we aimed to determine to what extent this virus utilizes the protein kinase D pathway during a nonsynchronized infection. Several molecular protein kinase D (PKD) regulators were targeted by RNA interference and viral production monitored. Surprisingly, many of these modulators negatively impacted the extracellular release of the virus. Overexpression studies, the use of pharmacological reagents, and assays to monitor intracellular lipids implicated in the biology of PKD suggested that these effects were oddly independent of total intracellular diacylglycerol levels. Instead, mapping of the viral intermediates by electron microscopy suggested that some of these modulators could regulate distinct steps along the viral egress pathway, notably nuclear egress. Altogether, this suggests a more complex contribution of PKD to HSV-1 egress than originally anticipated and new research avenues to explore.IMPORTANCE Viruses are obligatory parasites that highjack numerous cellular functions. This is certainly true when it comes to transporting viral particles within the cell. Herpesviruses share the unique property of traveling through the two nuclear membranes by subsequent budding and fusion and acquiring their final envelope from a cellular organelle. Albeit disputed, the overall evidence from many laboratories points to the trans-Golgi network (TGN) as the source of that membrane. Moreover, past findings revealed that the host protein kinase D (PKD) plays an important role at that stage, which is significant given the known implication of that protein in vesicular transport. The present findings suggest that the PKD machinery not only affects the late stages of herpes simplex virus I egress but also modulates earlier steps, such as nuclear egress. This opens up new means to control these viruses.
Keywords: Arf; CERT; GGA1; HSV; Nir2; PKD; arfaptin; egress; protein kinase D; transport.
Copyright © 2018 American Society for Microbiology.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

