Robust Human and Murine Hepatocyte Culture Models of Hepatitis B Virus Infection and Replication
- PMID: 30232184
- PMCID: PMC6232483
- DOI: 10.1128/JVI.01255-18
Robust Human and Murine Hepatocyte Culture Models of Hepatitis B Virus Infection and Replication
Abstract
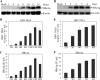
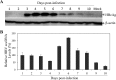
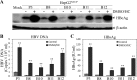
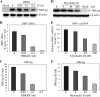
Hepatitis B virus (HBV) is a major cause of chronic liver diseases, including hepatitis, cirrhosis, and hepatocellular carcinoma. HBV research has been hampered by the lack of robust cell culture and small animal models of HBV infection. The discovery of sodium taurocholate cotransporting polypeptide (NTCP) as an HBV receptor has been a landmark advance in HBV research in recent years. Ectopic expression of NTCP in nonpermissive HepG2, Huh7, and AML12 cell lines confers HBV susceptibility. However, HBV replication in these human and murine hepatocyte cell lines appeared suboptimal. In the present study, we constructed stable NTCP-expressing HepG2 and AML12 cell lines and found that HBV permissiveness is correlated with NTCP expression. More significantly, we developed robust HBV cell culture models by treating the HBV-infected cells with dimethyl sulfoxide (DMSO) and hydrocortisone, which significantly promoted HBV replication and production. Mechanistic studies suggested that hydrocortisone significantly enhanced the transcription and expression of PGC1α and HNF4α, which are known to promote HBV transcription and replication. These new human and murine hepatocyte culture systems of HBV infection and replication will accelerate the determination of molecular aspects underlying HBV infection, replication, and morphogenesis in human and murine hepatocytes. We anticipate that our HBV cell culture models will also facilitate the discovery and development of antiviral drugs towards the ultimate eradication of chronic hepatitis B virus infection.IMPORTANCE HBV research has been greatly hampered by the lack of robust cell culture and small animal models of HBV infection and propagation. The discovery of NTCP as an HBV receptor has greatly impacted the field of HBV research. Although HBV infection of NTCP-expressing human and murine hepatocyte cell lines has been demonstrated, its replication in cell culture appeared inefficient. To further improve cell culture systems of HBV infection and replication, we constructed NTCP-expressing HepG2 and AML12 cell lines that are highly permissive to HBV infection. More significantly, we found that DMSO and hydrocortisone markedly enhanced HBV transcription and replication in human and murine hepatocytes when added to the cell culture medium. These new cell culture models of HBV infection and replication will facilitate HBV research and antiviral drug discovery towards the ultimate elimination of chronic hepatitis B virus infection.
Keywords: AML12; DMSO; HBV; HepG2; NTCP; PHH; hepatitis B virus; hydrocortisone; infection; morphogenesis; replication.
Copyright © 2018 American Society for Microbiology.
Figures
Similar articles
-
Unusual Features of Sodium Taurocholate Cotransporting Polypeptide as a Hepatitis B Virus Receptor.J Virol. 2016 Aug 26;90(18):8302-13. doi: 10.1128/JVI.01153-16. Print 2016 Sep 15. J Virol. 2016. PMID: 27384660 Free PMC article.
-
Evaluation and identification of hepatitis B virus entry inhibitors using HepG2 cells overexpressing a membrane transporter NTCP.Biochem Biophys Res Commun. 2014 Jan 17;443(3):808-13. doi: 10.1016/j.bbrc.2013.12.052. Epub 2013 Dec 14. Biochem Biophys Res Commun. 2014. PMID: 24342612
-
In Vitro Infection with Hepatitis B Virus Using Differentiated Human Serum Culture of Huh7.5-NTCP Cells without Requiring Dimethyl Sulfoxide.Viruses. 2021 Jan 12;13(1):97. doi: 10.3390/v13010097. Viruses. 2021. PMID: 33445753 Free PMC article.
-
HBV culture and infectious systems.Hepatol Int. 2016 Jul;10(4):559-66. doi: 10.1007/s12072-016-9712-y. Epub 2016 Mar 2. Hepatol Int. 2016. PMID: 26935052 Review.
-
NTCP opens the door for hepatitis B virus infection.Antiviral Res. 2015 Sep;121:24-30. doi: 10.1016/j.antiviral.2015.06.002. Epub 2015 Jun 10. Antiviral Res. 2015. PMID: 26071008 Review.
Cited by
-
Hepatitis B virus entry, assembly, and egress.Microbiol Mol Biol Rev. 2024 Dec 18;88(4):e0001424. doi: 10.1128/mmbr.00014-24. Epub 2024 Oct 23. Microbiol Mol Biol Rev. 2024. PMID: 39440957 Review.
-
New Insights on Molecular Mechanism of Hepatitis B Virus Covalently Closed Circular DNA Formation.Cells. 2020 Nov 6;9(11):2430. doi: 10.3390/cells9112430. Cells. 2020. PMID: 33172220 Free PMC article. Review.
-
Human low-density lipoprotein receptor plays an important role in hepatitis B virus infection.PLoS Pathog. 2021 Jul 22;17(7):e1009722. doi: 10.1371/journal.ppat.1009722. eCollection 2021 Jul. PLoS Pathog. 2021. PMID: 34293069 Free PMC article.
-
Hepatitis B Virus X Protein Stimulates Hepatitis C Virus (HCV) Replication by Protecting HCV Core Protein from E6AP-Mediated Proteasomal Degradation.Microbiol Spectr. 2022 Dec 21;10(6):e0143222. doi: 10.1128/spectrum.01432-22. Epub 2022 Nov 14. Microbiol Spectr. 2022. PMID: 36374094 Free PMC article.
-
Pathogenicity and virulence of Hepatitis B virus.Virulence. 2022 Dec;13(1):258-296. doi: 10.1080/21505594.2022.2028483. Virulence. 2022. PMID: 35100095 Free PMC article. Review.
References
-
- Zeisel MB, Lucifora J, Mason WS, Sureau C, Beck J, Levrero M, Kann M, Knolle PA, Benkirane M, Durantel D, Michel ML, Autran B, Cosset FL, Strick-Marchand H, Trepo C, Kao JH, Carrat F, Lacombe K, Schinazi RF, Barre-Sinoussi F, Delfraissy JF, Zoulim F. 2015. Towards an HBV cure: state-of-the-art and unresolved questions—report of the ANRS workshop on HBV cure. Gut 64:1314–1326. doi:10.1136/gutjnl-2014-308943. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

