Natural Secretory Immunoglobulins Promote Enteric Viral Infections
- PMID: 30232191
- PMCID: PMC6232472
- DOI: 10.1128/JVI.00826-18
Natural Secretory Immunoglobulins Promote Enteric Viral Infections
Abstract
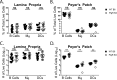
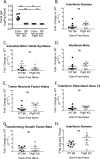
Noroviruses are enteric pathogens causing significant morbidity, mortality, and economic losses worldwide. Secretory immunoglobulins (sIg) are a first line of mucosal defense against enteric pathogens. They are secreted into the intestinal lumen via the polymeric immunoglobulin receptor (pIgR), where they bind to antigens. However, whether natural sIg protect against norovirus infection remains unknown. To determine if natural sIg alter murine norovirus (MNV) pathogenesis, we infected pIgR knockout (KO) mice, which lack sIg in mucosal secretions. Acute MNV infection was significantly reduced in pIgR KO mice compared to controls, despite increased MNV target cells in the Peyer's patch. Natural sIg did not alter MNV binding to the follicle-associated epithelium (FAE) or crossing of the FAE into the lymphoid follicle. Instead, naive pIgR KO mice had enhanced levels of the antiviral inflammatory molecules interferon gamma (IFN-γ) and inducible nitric oxide synthase (iNOS) in the ileum compared to controls. Strikingly, depletion of the intestinal microbiota in pIgR KO and control mice resulted in comparable IFN-γ and iNOS levels, as well as MNV infectious titers. IFN-γ treatment of wild-type (WT) mice and neutralization of IFN-γ in pIgR KO mice modulated MNV titers, implicating the antiviral cytokine in the phenotype. Reduced gastrointestinal infection in pIgR KO mice was also observed with another enteric virus, reovirus. Collectively, our findings suggest that natural sIg are not protective during enteric virus infection, but rather, that sIg promote enteric viral infection through alterations in microbial immune responses.IMPORTANCE Enteric virus, such as norovirus, infections cause significant morbidity and mortality worldwide. However, direct antiviral infection prevention strategies are limited. Blocking host entry and initiation of infection provides an established avenue for intervention. Here, we investigated the role of the polymeric immunoglobulin receptor (pIgR)-secretory immunoglobulin (sIg) cycle during enteric virus infections. The innate immune functions of sIg (agglutination, immune exclusion, neutralization, and expulsion) were not required during control of acute murine norovirus (MNV) infection. Instead, lack of pIgR resulted in increased IFN-γ levels, which contributed to reduced MNV titers. Another enteric virus, reovirus, also showed decreased infection in pIgR KO mice. Collectively, our data point to a model in which sIg-mediated microbial sensing promotes norovirus and reovirus infection. These data provide the first evidence of the proviral role of natural sIg during enteric virus infections and provide another example of how intestinal bacterial communities indirectly influence MNV pathogenesis.
Keywords: RNA virus; enteric viruses; gastrointestinal infection; pathogenesis.
Copyright © 2018 American Society for Microbiology.
Figures
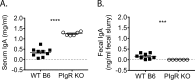
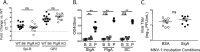
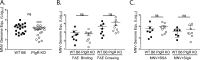





Similar articles
-
Expression of Ifnlr1 on Intestinal Epithelial Cells Is Critical to the Antiviral Effects of Interferon Lambda against Norovirus and Reovirus.J Virol. 2017 Mar 13;91(7):e02079-16. doi: 10.1128/JVI.02079-16. Print 2017 Apr 1. J Virol. 2017. PMID: 28077655 Free PMC article.
-
Efficient norovirus and reovirus replication in the mouse intestine requires microfold (M) cells.J Virol. 2014 Jun;88(12):6934-43. doi: 10.1128/JVI.00204-14. Epub 2014 Apr 2. J Virol. 2014. PMID: 24696493 Free PMC article.
-
Oral Norovirus Infection Is Blocked in Mice Lacking Peyer's Patches and Mature M Cells.J Virol. 2015 Nov 18;90(3):1499-506. doi: 10.1128/JVI.02872-15. Print 2016 Feb 1. J Virol. 2015. PMID: 26581993 Free PMC article.
-
The Role of the Polymeric Immunoglobulin Receptor and Secretory Immunoglobulins during Mucosal Infection and Immunity.Viruses. 2018 May 3;10(5):237. doi: 10.3390/v10050237. Viruses. 2018. PMID: 29751532 Free PMC article. Review.
-
The Role of Interferon in Persistent Viral Infection: Insights from Murine Norovirus.Trends Microbiol. 2018 Jun;26(6):510-524. doi: 10.1016/j.tim.2017.10.010. Epub 2017 Nov 17. Trends Microbiol. 2018. PMID: 29157967 Free PMC article. Review.
Cited by
-
Virus interactions with bacteria: Partners in the infectious dance.PLoS Pathog. 2020 Feb 11;16(2):e1008234. doi: 10.1371/journal.ppat.1008234. eCollection 2020 Feb. PLoS Pathog. 2020. PMID: 32045465 Free PMC article. Review. No abstract available.
-
A single mutation in an enteric virus alters tropism and sensitivity to microbiota.Proc Natl Acad Sci U S A. 2025 Apr 22;122(16):e2500612122. doi: 10.1073/pnas.2500612122. Epub 2025 Apr 16. Proc Natl Acad Sci U S A. 2025. PMID: 40238456
-
IgA and FcαRI: Pathological Roles and Therapeutic Opportunities.Front Immunol. 2019 Mar 22;10:553. doi: 10.3389/fimmu.2019.00553. eCollection 2019. Front Immunol. 2019. PMID: 30984170 Free PMC article. Review.
-
The Antigenic Topology of Norovirus as Defined by B and T Cell Epitope Mapping: Implications for Universal Vaccines and Therapeutics.Viruses. 2019 May 10;11(5):432. doi: 10.3390/v11050432. Viruses. 2019. PMID: 31083353 Free PMC article. Review.
-
Diverse Mechanisms Underlie Enhancement of Enteric Viruses by the Mammalian Intestinal Microbiota.Viruses. 2019 Aug 17;11(8):760. doi: 10.3390/v11080760. Viruses. 2019. PMID: 31426458 Free PMC article. Review.
References
-
- Davids BJ, Palm JE, Housley MP, Smith JR, Andersen YS, Martin MG, Hendrickson BA, Johansen FE, Svard SG, Gillin FD, Eckmann L. 2006. Polymeric immunoglobulin receptor in intestinal immune defense against the lumen-dwelling protozoan parasite Giardia. J Immunol 177:6281–6290. doi:10.4049/jimmunol.177.9.6281. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

