Axonal organization defects in the hippocampus of adult conditional BACE1 knockout mice
- PMID: 30232227
- PMCID: PMC11017370
- DOI: 10.1126/scitranslmed.aao5620
Axonal organization defects in the hippocampus of adult conditional BACE1 knockout mice
Abstract
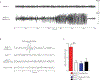
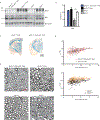
β-Site APP (amyloid precursor protein) cleaving enzyme 1 (BACE1) is the β-secretase enzyme that initiates production of the toxic amyloid-β peptide that accumulates in the brains of patients with Alzheimer's disease (AD). Hence, BACE1 is a prime therapeutic target, and several BACE1 inhibitor drugs are currently being tested in clinical trials for AD. However, the safety of BACE1 inhibition is unclear. Germline BACE1 knockout mice have multiple neurological phenotypes, although these could arise from BACE1 deficiency during development. To address this question, we report that tamoxifen-inducible conditional BACE1 knockout mice in which the Bace1 gene was ablated in the adult largely lacked the phenotypes observed in germline BACE1 knockout mice. However, one BACE1-null phenotype was induced after Bace1 gene deletion in the adult mouse brain. This phenotype showed reduced length and disorganization of the hippocampal mossy fiber infrapyramidal bundle, the axonal pathway of dentate gyrus granule cells that is maintained by neurogenesis in the mouse brain. This defect in axonal organization correlated with reduced BACE1-mediated cleavage of the neural cell adhesion protein close homolog of L1 (CHL1), which has previously been associated with axon guidance. Although our results indicate that BACE1 inhibition in the adult mouse brain may avoid phenotypes associated with BACE1 deficiency during embryonic and postnatal development, they also suggest that BACE1 inhibitor drugs developed for treating AD may disrupt the organization of an axonal pathway in the hippocampus, an important structure for learning and memory.
Copyright © 2018 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Conflict of interest statement
Figures



References
-
- Tanzi RE, Gusella JF, Watkins PC, Bruns GA, St George-Hyslop P, Van Keuren ML, Patterson D, Pagan S, Kurnit DM, Neve RL, Amyloid beta protein gene: cDNA, mRNA distribution, and genetic linkage near the Alzheimer locus. Science 235, 880–884 (1987). - PubMed
-
- Vassar R, Bennett BD, Babu-Khan S, Kahn S, Mendiaz EA, Denis P, Teplow DB, Ross S, Amarante P, Loeloff R, Luo Y, Fisher S, Fuller J, Edenson S, Lile J, Jarosinski MA, Biere AL, Curran E, Burgess T, Louis JC, Collins F, Treanor J, Rogers G, Citron M, Beta-secretase cleavage of Alzheimer’s amyloid precursor protein by the transmembrane aspartic protease BACE. Science 286, 735–741 (1999). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

