Substance P and IL-33 administered together stimulate a marked secretion of IL-1β from human mast cells, inhibited by methoxyluteolin
- PMID: 30232261
- PMCID: PMC6176605
- DOI: 10.1073/pnas.1810133115
Substance P and IL-33 administered together stimulate a marked secretion of IL-1β from human mast cells, inhibited by methoxyluteolin
Abstract
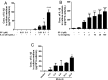
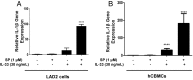
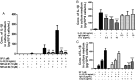
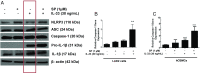
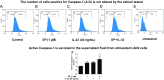
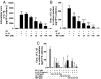
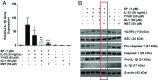
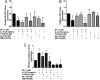
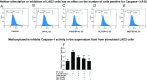
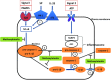
Mast cells are critical for allergic and inflammatory responses in which the peptide substance P (SP) and the cytokine IL-33 are involved. SP (0.01-1 μM) administered together with IL-33 (30 ng/mL) to human cultured LAD2 mast cells stimulates a marked increase (P < 0.0001) in secretion of the proinflammatory cytokine IL-1β. Preincubation of LAD2 (30 min) with the SP receptor (NK-1) antagonists L-733,060 (10 μM) or CP-96345 (10 µM) inhibits (P < 0.001) secretion of IL-1β stimulated by either SP (1 μM) or SP together with IL-33 (30 ng/mL). Surprisingly, secretion of IL-1β stimulated by IL-33 is inhibited (P < 0.001) by each NK-1 antagonist. Preincubation with an antibody against the IL-33 receptor ST2 inhibits (P < 0.0001) secretion of IL-1β stimulated either by IL-33 or together with SP. The combination of SP (1 μM) with IL-33 (30 ng/mL) increases IL-1β gene expression by 90-fold in LAD2 cells and by 200-fold in primary cultured mast cells from human umbilical cord blood. The combination of SP and IL-33 increases intracellular levels of IL-1β in LAD2 by 100-fold and gene expression of IL-1β and procaspase-1 by fivefold and pro-IL-1β by twofold. Active caspase-1 is present even in unstimulated cells and is detected extracellularly. Preincubation of LAD2 cells with the natural flavonoid methoxyluteolin (1-100 mM) inhibits (P < 0.0001) secretion and gene expression of IL-1β, procaspase-1, and pro-IL-1β. Mast cell secretion of IL-1β in response to SP and IL-33 reveals targets for the development of antiinflammatory therapies.
Keywords: IL-1β; IL-33; inflammation; mast cells; substance P.
Conflict of interest statement
Conflict of interest statement: T.C.T. is the recipient of US patent no. 7,906,153 covering the use of flavonoids in neuroinflammatory conditions.
Figures
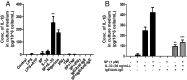
Similar articles
-
SP and IL-33 together markedly enhance TNF synthesis and secretion from human mast cells mediated by the interaction of their receptors.Proc Natl Acad Sci U S A. 2017 May 16;114(20):E4002-E4009. doi: 10.1073/pnas.1524845114. Epub 2017 May 1. Proc Natl Acad Sci U S A. 2017. PMID: 28461492 Free PMC article.
-
Chondroitin sulfate inhibits secretion of TNF and CXCL8 from human mast cells stimulated by IL-33.Biofactors. 2019 Jan;45(1):49-61. doi: 10.1002/biof.1464. Epub 2018 Dec 6. Biofactors. 2019. PMID: 30521103
-
IL-33 stimulates human mast cell release of CCL5 and CCL2 via MAPK and NF-κB, inhibited by methoxyluteolin.Eur J Pharmacol. 2019 Dec 15;865:172760. doi: 10.1016/j.ejphar.2019.172760. Epub 2019 Oct 26. Eur J Pharmacol. 2019. PMID: 31669588
-
Interleukin 33 and interleukin 4 regulate interleukin 31 gene expression and secretion from human laboratory of allergic diseases 2 mast cells stimulated by substance P and/or immunoglobulin E.Allergy Asthma Proc. 2018 Mar 1;39(2):153-160. doi: 10.2500/aap.2018.38.4105. Allergy Asthma Proc. 2018. PMID: 29490771 Free PMC article.
-
Mast Cells, Neuroinflammation and Pain in Fibromyalgia Syndrome.Front Cell Neurosci. 2019 Aug 2;13:353. doi: 10.3389/fncel.2019.00353. eCollection 2019. Front Cell Neurosci. 2019. PMID: 31427928 Free PMC article. Review.
Cited by
-
The IL-33/ST2 axis is protective against acute inflammation during the course of periodontitis.Nat Commun. 2024 Mar 28;15(1):2707. doi: 10.1038/s41467-024-46746-2. Nat Commun. 2024. PMID: 38548743 Free PMC article.
-
The Chemistry and the Anti-Inflammatory Activity of Polymethoxyflavonoids from Citrus Genus.Antioxidants (Basel). 2022 Dec 22;12(1):23. doi: 10.3390/antiox12010023. Antioxidants (Basel). 2022. PMID: 36670885 Free PMC article. Review.
-
Potential Role of Moesin in Regulating Mast Cell Secretion.Int J Mol Sci. 2023 Jul 28;24(15):12081. doi: 10.3390/ijms241512081. Int J Mol Sci. 2023. PMID: 37569454 Free PMC article. Review.
-
Ways to Address Perinatal Mast Cell Activation and Focal Brain Inflammation, including Response to SARS-CoV-2, in Autism Spectrum Disorder.J Pers Med. 2021 Aug 29;11(9):860. doi: 10.3390/jpm11090860. J Pers Med. 2021. PMID: 34575637 Free PMC article. Review.
-
Involvement of Mast Cells in the Pathophysiology of Pain.Front Cell Neurosci. 2021 Jun 10;15:665066. doi: 10.3389/fncel.2021.665066. eCollection 2021. Front Cell Neurosci. 2021. PMID: 34177465 Free PMC article.
References
-
- Rodewald HR, Dessing M, Dvorak AM, Galli SJ. Identification of a committed precursor for the mast cell lineage. Science. 1996;271:818–822. - PubMed
-
- Schmetzer O, Valentin P, Church MK, Maurer M, Siebenhaar F. Murine and human mast cell progenitors. Eur J Pharmacol. 2016;778:2–10. - PubMed
-
- Theoharides TC, Valent P, Akin C. Mast cells, mastocytosis, and related disorders. N Engl J Med. 2015;373:163–172. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

