Beclin-1 regulates cigarette smoke-induced kidney injury in a murine model of chronic obstructive pulmonary disease
- PMID: 30232271
- PMCID: PMC6237223
- DOI: 10.1172/jci.insight.99592
Beclin-1 regulates cigarette smoke-induced kidney injury in a murine model of chronic obstructive pulmonary disease
Abstract
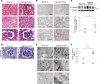
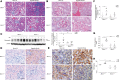
Chronic obstructive pulmonary disease (COPD), associated with cigarette smoke-induced (CS-induced) emphysema, contributes significantly to the global health care burden of disease. Although chronic kidney disease (CKD) may occur in patients with COPD, the relationship between COPD and CKD remains unclear. Using a murine model of experimental COPD, we show that chronic CS exposure resulted in marked kidney injury and fibrosis, as evidenced by histological and ultrastructural changes, altered macrophage subpopulations, and expression of tissue injury, fibrosis, and oxidative stress markers. CS induced mitochondrial dysfunction, and increased autophagic flux in kidney tissues and in kidney tubular epithelial (HK-2) cells, as determined by LC3B turnover assays. Mice heterozygous for Beclin-1 (Becn1+/-) were protected from the development of kidney tissue injury and renal fibrosis in response to CS exposure, and displayed impaired basal and inducible mitochondrial turnover by mitophagy. Interestingly, CS caused a reduction of Beclin-1 expression in mouse kidneys and kidney tubular epithelial cells, attributed to increased autophagy-dependent turnover of Beclin-1. These results suggest that Beclin-1 is required for CS-induced kidney injury and that reduced levels of Beclin-1 may confer renoprotection. These results identify the kidney as a target for CS-induced injury in COPD and the Beclin-1-dependent autophagy pathway as a potential therapeutic target in CKD.
Keywords: Autophagy; COPD; Chronic kidney disease; Nephrology; Pulmonology.
Conflict of interest statement
Figures





References
-
- US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics. Health, United States, 2016: With Chartbook on Long-Term Trends in Health. Hyattsville, Maryland, USA: National Center for Health Statistics; 2017. https://www.cdc.gov/nchs/data/hus/hus16.pdf Accessed September 7, 2018.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

