Abnormal neutrophil signature in the blood and pancreas of presymptomatic and symptomatic type 1 diabetes
- PMID: 30232284
- PMCID: PMC6237216
- DOI: 10.1172/jci.insight.122146
Abnormal neutrophil signature in the blood and pancreas of presymptomatic and symptomatic type 1 diabetes
Abstract
Background: Neutrophils and their inflammatory mediators are key pathogenic components in multiple autoimmune diseases, while their role in human type 1 diabetes (T1D), a disease that progresses sequentially through identifiable stages prior to the clinical onset, is not well understood. We previously reported that the number of circulating neutrophils is reduced in patients with T1D and in presymptomatic at-risk subjects. The aim of the present work was to identify possible changes in circulating and pancreas-residing neutrophils throughout the disease course to better elucidate neutrophil involvement in human T1D.
Methods: Data collected from 389 subjects at risk of developing T1D, and enrolled in 4 distinct studies performed by TrialNet, were analyzed with comprehensive statistical approaches to determine whether the number of circulating neutrophils correlates with pancreas function. To obtain a broad analysis of pancreas-infiltrating neutrophils throughout all disease stages, pancreas sections collected worldwide from 4 different cohorts (i.e., nPOD, DiViD, Siena, and Exeter) were analyzed by immunohistochemistry and immunofluorescence. Finally, circulating neutrophils were purified from unrelated nondiabetic subjects and donors at various T1D stages and their transcriptomic signature was determined by RNA sequencing.
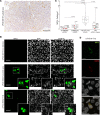
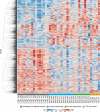
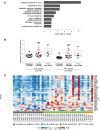
Results: Here, we show that the decline in β cell function is greatest in individuals with the lowest peripheral neutrophil numbers. Neutrophils infiltrate the pancreas prior to the onset of symptoms and they continue to do so as the disease progresses. Of interest, a fraction of these pancreas-infiltrating neutrophils also extrudes neutrophil extracellular traps (NETs), suggesting a tissue-specific pathogenic role. Whole-transcriptome analysis of purified blood neutrophils revealed a unique molecular signature that is distinguished by an overabundance of IFN-associated genes; despite being healthy, said signature is already present in T1D-autoantibody-negative at-risk subjects.
Conclusions: These results reveal an unexpected abnormality in neutrophil disposition both in the circulation and in the pancreas of presymptomatic and symptomatic T1D subjects, implying that targeting neutrophils might represent a previously unrecognized therapeutic modality.
Funding: Juvenile Diabetes Research Foundation (JDRF), NIH, Diabetes UK.
Keywords: Autoimmune diseases; Autoimmunity; Immunology; Innate immunity.
Conflict of interest statement
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
- U01 DK085476/DK/NIDDK NIH HHS/United States
- U01 DK061010/DK/NIDDK NIH HHS/United States
- 15/0005156/DUK_/Diabetes UK/United Kingdom
- U01 DK085453/DK/NIDDK NIH HHS/United States
- U01 DK103282/DK/NIDDK NIH HHS/United States
- UC4 DK106993/DK/NIDDK NIH HHS/United States
- U01 DK061042/DK/NIDDK NIH HHS/United States
- U01 DK085509/DK/NIDDK NIH HHS/United States
- P30 DK017047/DK/NIDDK NIH HHS/United States
- U01 DK085466/DK/NIDDK NIH HHS/United States
- U01 DK103153/DK/NIDDK NIH HHS/United States
- U01 DK061058/DK/NIDDK NIH HHS/United States
- U01 DK106984/DK/NIDDK NIH HHS/United States
- U01 DK085499/DK/NIDDK NIH HHS/United States
- U01 DK107013/DK/NIDDK NIH HHS/United States
- U01 DK103266/DK/NIDDK NIH HHS/United States
- U01 DK107014/DK/NIDDK NIH HHS/United States
- U01 DK106994/DK/NIDDK NIH HHS/United States
- U01 DK061034/DK/NIDDK NIH HHS/United States
- U01 DK085461/DK/NIDDK NIH HHS/United States
- U01 DK103180/DK/NIDDK NIH HHS/United States
- U01 DK085465/DK/NIDDK NIH HHS/United States
- U01 DK085504/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

