Single-cell RNA sequencing reveals microglia-like cells in cerebrospinal fluid during virologically suppressed HIV
- PMID: 30232286
- PMCID: PMC6237230
- DOI: 10.1172/jci.insight.121718
Single-cell RNA sequencing reveals microglia-like cells in cerebrospinal fluid during virologically suppressed HIV
Abstract
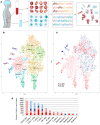
Central nervous system (CNS) immune activation is an important driver of neuronal injury during several neurodegenerative and neuroinflammatory diseases. During HIV infection, CNS immune activation is associated with high rates of neurocognitive impairment, even during sustained long-term suppressive antiretroviral therapy (ART). However, the cellular subsets that drive immune activation and neuronal damage in the CNS during HIV infection and other neurological conditions remain unknown, in part because CNS cells are difficult to access in living humans. Using single-cell RNA sequencing (scRNA-seq) on cerebrospinal fluid (CSF) and blood from adults with and without HIV, we identified a rare (<5% of cells) subset of myeloid cells that are found only in CSF and that present a gene expression signature that overlaps significantly with neurodegenerative disease-associated microglia. This highlights the power of scRNA-seq of CSF to identify rare CNS immune cell subsets that may perpetuate neuronal injury during HIV infection and other conditions.
Keywords: AIDS/HIV; Inflammation; Neurological disorders.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

