Haptoglobin improves shock, lung injury, and survival in canine pneumonia
- PMID: 30232287
- PMCID: PMC6237235
- DOI: 10.1172/jci.insight.123013
Haptoglobin improves shock, lung injury, and survival in canine pneumonia
Abstract
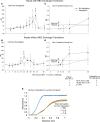
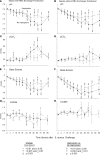
During the last half-century, numerous antiinflammatory agents were tested in dozens of clinical trials and have proven ineffective for treating septic shock. The observation in multiple studies that cell-free hemoglobin (CFH) levels are elevated during clinical sepsis and that the degree of increase correlates with higher mortality suggests an alternative approach. Human haptoglobin binds CFH with high affinity and, therefore, can potentially reduce iron availability and oxidative activity. CFH levels are elevated over approximately 24-48 hours in our antibiotic-treated canine model of S. aureus pneumonia that simulates the cardiovascular abnormalities of human septic shock. In this 96-hour model, resuscitative treatments, mechanical ventilation, sedation, and continuous care are translatable to management in human intensive care units. We found, in this S. aureus pneumonia model inducing septic shock, that commercial human haptoglobin concentrate infusions over 48-hours bind canine CFH, increase CFH clearance, and lower circulating iron. Over the 96-hour study, this treatment was associated with an improved metabolic profile (pH, lactate), less lung injury, reversal of shock, and increased survival. Haptoglobin binding compartmentalized CFH to the intravascular space. This observation, in combination with increasing CFHs clearance, reduced available iron as a potential source of bacterial nutrition while decreasing the ability for CFH and iron to cause extravascular oxidative tissue injury. In contrast, haptoglobin therapy had no measurable antiinflammatory effect on elevations in proinflammatory C-reactive protein and cytokine levels. Haptoglobin therapy enhances normal host defense mechanisms in contrast to previously studied antiinflammatory sepsis therapies, making it a biologically plausible novel approach to treat septic shock.
Keywords: Bacterial infections; Clinical Trials; Drug therapy; Infectious disease; Innate immunity.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

