Clearance of senescent glial cells prevents tau-dependent pathology and cognitive decline
- PMID: 30232451
- PMCID: PMC6206507
- DOI: 10.1038/s41586-018-0543-y
Clearance of senescent glial cells prevents tau-dependent pathology and cognitive decline
Abstract
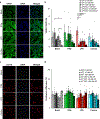
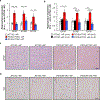
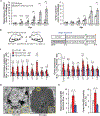
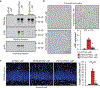
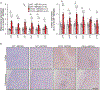
Cellular senescence, which is characterized by an irreversible cell-cycle arrest1 accompanied by a distinctive secretory phenotype2, can be induced through various intracellular and extracellular factors. Senescent cells that express the cell cycle inhibitory protein p16INK4A have been found to actively drive naturally occurring age-related tissue deterioration3,4 and contribute to several diseases associated with ageing, including atherosclerosis5 and osteoarthritis6. Various markers of senescence have been observed in patients with neurodegenerative diseases7-9; however, a role for senescent cells in the aetiology of these pathologies is unknown. Here we show a causal link between the accumulation of senescent cells and cognition-associated neuronal loss. We found that the MAPTP301SPS19 mouse model of tau-dependent neurodegenerative disease10 accumulates p16INK4A-positive senescent astrocytes and microglia. Clearance of these cells as they arise using INK-ATTAC transgenic mice prevents gliosis, hyperphosphorylation of both soluble and insoluble tau leading to neurofibrillary tangle deposition, and degeneration of cortical and hippocampal neurons, thus preserving cognitive function. Pharmacological intervention with a first-generation senolytic modulates tau aggregation. Collectively, these results show that senescent cells have a role in the initiation and progression of tau-mediated disease, and suggest that targeting senescent cells may provide a therapeutic avenue for the treatment of these pathologies.
Figures
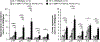
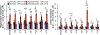



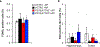



Comment in
-
Elimination of senescent cells prevents neurodegeneration in mice.Nature. 2018 Oct;562(7728):503-504. doi: 10.1038/d41586-018-06677-7. Nature. 2018. PMID: 30349121 No abstract available.
References
-
- Hayflick L & Moorhead PS The serial cultivation of human diploid cell strains. Exp Cell Res 25, 585–621 (1961). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

