Ring nucleases deactivate type III CRISPR ribonucleases by degrading cyclic oligoadenylate
- PMID: 30232454
- PMCID: PMC6219705
- DOI: 10.1038/s41586-018-0557-5
Ring nucleases deactivate type III CRISPR ribonucleases by degrading cyclic oligoadenylate
Abstract
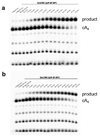
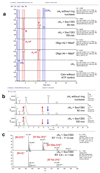
The CRISPR system provides adaptive immunity against mobile genetic elements in prokaryotes, using small CRISPR RNAs that direct effector complexes to degrade invading nucleic acids1-3. Type III effector complexes were recently demonstrated to synthesize a novel second messenger, cyclic oligoadenylate, on binding target RNA4,5. Cyclic oligoadenylate, in turn, binds to and activates ribonucleases and other factors-via a CRISPR-associated Rossman-fold domain-and thereby induces in the cell an antiviral state that is important for immunity. The mechanism of the 'off-switch' that resets the system is not understood. Here we identify the nuclease that degrades these cyclic oligoadenylate ring molecules. This 'ring nuclease' is itself a protein of the CRISPR-associated Rossman-fold family, and has a metal-independent mechanism that cleaves cyclic tetraadenylate rings to generate linear diadenylate species and switches off the antiviral state. The identification of ring nucleases adds an important insight to the CRISPR system.
Conflict of interest statement
Figures










Comment in
-
If You'd Like to Stop a Type III CRISPR Ribonuclease, Then You Should Put a Ring (Nuclease) on It.Mol Cell. 2018 Nov 15;72(4):608-609. doi: 10.1016/j.molcel.2018.10.048. Mol Cell. 2018. PMID: 30444997
References
-
- Mojica FJ, Rodriguez-Valera F. The discovery of CRISPR in archaea and bacteria. FEBS J. 2016;283:3162–3169. - PubMed
-
- Niewoehner O, et al. Type III CRISPR-Cas systems produce cyclic oligoadenylate second messengers. Nature. 2017;548:543–548. - PubMed
-
- Kazlauskiene M, Kostiuk G, Venclovas C, Tamulaitis G, Siksnys V. A cyclic oligonucleotide signaling pathway in type III CRISPR-Cas systems. Science. 2017;357:605–609. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

