Magnolol dimer-derived fragments as PPARγ-selective probes
- PMID: 30232493
- PMCID: PMC6180429
- DOI: 10.1039/c8ob01745j
Magnolol dimer-derived fragments as PPARγ-selective probes
Abstract
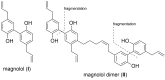
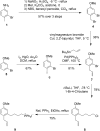
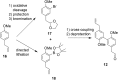
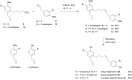
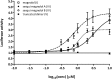
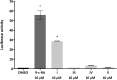
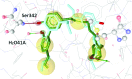
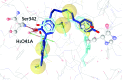
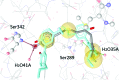
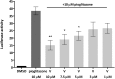
Partial agonists of the transcription factor PPARγ (peroxisome proliferator-activated receptor γ) have shown potential for the treatment of metabolic and inflammatory conditions and novel activators serve as valuable tool and lead compounds. Based on the natural product magnolol (I) and recent structural information of the ligand-target interaction we have previously developed magnolol dimer (II) which has been shown to have enhanced affinity towards PPARγ and improved selectivity over RXRα (retinoid X receptor α), PPARγ's heterodimerization partner. In this contribution we report the synthesis and evaluation of three fragments of the dimeric lead compound by structural simplifications. Sesqui magnolol A and B (III and IV) were found to exhibit comparable activities to magnolol dimer (II) and selectivity over RXRα persisted. Computational studies suggest a common pharmacophore of the distinctive biphenyl motifs. Truncated magnolol dimer (V) on the other hand does not share this feature and was found to act as an antagonist.
Figures


Similar articles
-
Linked magnolol dimer as a selective PPARγ agonist - Structure-based rational design, synthesis, and bioactivity evaluation.Sci Rep. 2017 Oct 20;7(1):13002. doi: 10.1038/s41598-017-12628-5. Sci Rep. 2017. PMID: 29057944 Free PMC article.
-
Molecular determinants of magnolol targeting both RXRα and PPARγ.PLoS One. 2011;6(11):e28253. doi: 10.1371/journal.pone.0028253. Epub 2011 Nov 29. PLoS One. 2011. PMID: 22140563 Free PMC article.
-
Discovery and SAR of Natural-Product-Inspired RXR Agonists with Heterodimer Selectivity to PPARδ-RXR.ACS Chem Biol. 2020 Jun 19;15(6):1526-1534. doi: 10.1021/acschembio.0c00146. Epub 2020 May 6. ACS Chem Biol. 2020. PMID: 32374156
-
The antiplatelet activity of magnolol is mediated by PPAR-β/γ.Biochem Pharmacol. 2012 Sep 15;84(6):793-803. doi: 10.1016/j.bcp.2012.06.022. Epub 2012 Jun 29. Biochem Pharmacol. 2012. PMID: 22750553
-
Natural product agonists of peroxisome proliferator-activated receptor gamma (PPARγ): a review.Biochem Pharmacol. 2014 Nov 1;92(1):73-89. doi: 10.1016/j.bcp.2014.07.018. Epub 2014 Jul 30. Biochem Pharmacol. 2014. PMID: 25083916 Free PMC article. Review.
Cited by
-
Identification of Plant Phenolics from Paulownia tomentosa and Morus alba as Novel PPARγ Partial Agonists and Hypoglycemic Agents.J Agric Food Chem. 2025 Jun 4;73(22):13960-13972. doi: 10.1021/acs.jafc.4c11398. Epub 2025 May 20. J Agric Food Chem. 2025. PMID: 40392982 Free PMC article.
-
Magnolol may contribute to barrier function improvement on imiquimod-induced psoriasis-like dermatitis animal model via the downregulation of interleukin-23.Exp Ther Med. 2021 May;21(5):448. doi: 10.3892/etm.2021.9876. Epub 2021 Mar 1. Exp Ther Med. 2021. PMID: 33747183 Free PMC article.
-
Magnolol Reduces Atopic Dermatitis-like Symptoms in BALB/c Mice.Life (Basel). 2024 Mar 5;14(3):339. doi: 10.3390/life14030339. Life (Basel). 2024. PMID: 38541664 Free PMC article.
References
-
- Lehrke M., Lazar M. A. Cell. 2005;123:993. - PubMed
-
- Rosen E. D., Sarraf P., Troy A. E., Bradwin G., Moore K., Milstone D. S., Spiegelman B. M., Mortensen R. M. Mol. Cell. 1999;4:611. - PubMed
-
- Gross B., Pawlak M., Lefebvre P., Staels B. Nat. Rev. Endocrinol. 2017;13:36. - PubMed
-
- Home P. Diabetes Care. 2011;34:215.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

