Inhibition of neurite outgrowth using commercial myelin associated glycoprotein-Fc in neuro-2a cells
- PMID: 30233061
- PMCID: PMC6183046
- DOI: 10.4103/1673-5374.239438
Inhibition of neurite outgrowth using commercial myelin associated glycoprotein-Fc in neuro-2a cells
Abstract
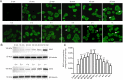
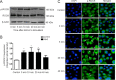
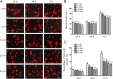
Myelin-associated glycoprotein (MAG) inhibits the growth of neurites from nerve cells. Extraction and purification of MAG require complex operations; therefore, we attempted to determine whether commercially available MAG-Fc can replace endogenous MAG for research purposes. Immunofluorescence using specific antibodies against MAG, Nogo receptor (NgR) and paired immunoglobulin-like receptor B (PirB) was used to determine whether MAG-Fc can be endocytosed by neuro-2a cells. In addition, neurite outgrowth of neuro-2a cells treated with different doses of MAG-Fc was evaluated. Enzyme linked immunosorbent assays were used to measure RhoA activity. Western blot assays were conducted to assess Rho-associated protein kinase (ROCK) phosphorylation. Neuro-2a cells expressed NgR and PirB, and MAG-Fc could be endocytosed by binding to NgR and PirB. This activated intracellular signaling pathways to increase RhoA activity and ROCK phosphorylation, ultimately inhibiting neurite outgrowth. These findings not only verify that MAG-Fc can inhibit the growth of neural neurites by activating RhoA signaling pathways, similarly to endogenous MAG, but also clearly demonstrate that commercial MAG-Fc is suitable for experimental studies of neurite outgrowth.
Keywords: MAG-Fc; RhoA/ROCK signaling pathways; cell culture; myelin growth inhibitors; myelin-associated glycoprotein; nerve regeneration; neural regeneration; neurite outgrowth; neuro-2a cell line; receptors for myelin-associated glycoprotein.
Conflict of interest statement
There are no conflicts of interest regarding financial interests, authorship, and copyright
Figures


Similar articles
-
LDL receptor-related protein-1 is a sialic-acid-independent receptor for myelin-associated glycoprotein that functions in neurite outgrowth inhibition by MAG and CNS myelin.J Cell Sci. 2013 Jan 1;126(Pt 1):209-20. doi: 10.1242/jcs.113191. Epub 2012 Nov 6. J Cell Sci. 2013. PMID: 23132925 Free PMC article.
-
Myelin-associated glycoprotein modulates apoptosis of motoneurons during early postnatal development via NgR/p75(NTR) receptor-mediated activation of RhoA signaling pathways.Cell Death Dis. 2015 Sep 3;6(9):e1876. doi: 10.1038/cddis.2015.228. Cell Death Dis. 2015. PMID: 26335717 Free PMC article.
-
Nogo-A and myelin-associated glycoprotein mediate neurite growth inhibition by antagonistic regulation of RhoA and Rac1.J Neurosci. 2002 Dec 1;22(23):10368-76. doi: 10.1523/JNEUROSCI.22-23-10368.2002. J Neurosci. 2002. PMID: 12451136 Free PMC article.
-
A hypothesis about the relationship of myelin-associated glycoprotein's function in myelinated axons to its capacity to inhibit neurite outgrowth.Neurochem Res. 2009 Jan;34(1):79-86. doi: 10.1007/s11064-008-9668-y. Epub 2008 Apr 12. Neurochem Res. 2009. PMID: 18408997 Review.
-
PirB is a novel potential therapeutic target for enhancing axonal regeneration and synaptic plasticity following CNS injury in mammals.J Drug Target. 2014 Jun;22(5):365-71. doi: 10.3109/1061186X.2013.878939. Epub 2014 Jan 9. J Drug Target. 2014. PMID: 24405091 Review.
Cited by
-
RhoA-GTPase Modulates Neurite Outgrowth by Regulating the Expression of Spastin and p60-Katanin.Cells. 2020 Jan 16;9(1):230. doi: 10.3390/cells9010230. Cells. 2020. PMID: 31963385 Free PMC article.
References
-
- Atwal JK, Pinkston-Gosse J, Syken J, Stawicki S, Wu Y, Shatz C, Tessier-Lavigne M. PirB is a functional receptor for myelin inhibitors of axonal regeneration. Science. 2008;322:967–970. - PubMed
-
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. - PubMed
-
- Cao Z, Gao Y, Deng K, Williams G, Doherty P, Walsh FS. Receptors for myelin inhibitors: Structures and therapeutic opportunities. Mol Cell Neurosci. 2010;43:1–14. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

