Cognitive deficits and Alzheimer-like neuropathological impairments during adolescence in a rat model of type 2 diabetes mellitus
- PMID: 30233075
- PMCID: PMC6183048
- DOI: 10.4103/1673-5374.239448
Cognitive deficits and Alzheimer-like neuropathological impairments during adolescence in a rat model of type 2 diabetes mellitus
Abstract
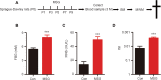
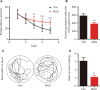
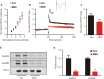
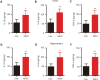
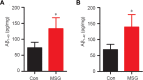
Numerous studies have shown that many patients who suffer from type 2 diabetes mellitus exhibit cognitive dysfunction and neuronal synaptic impairments. Therefore, growing evidence suggests that type 2 diabetes mellitus has a close relationship with occurrence and progression of neurodegeneration and neural impairment in Alzheimer's disease. However, the relationship between metabolic disorders caused by type 2 diabetes mellitus and neurodegeneration and neural impairments in Alzheimer's disease is still not fully determined. Thus, in this study, we replicated a type 2 diabetic animal model by subcutaneous injection of newborn Sprague-Dawley rats with monosodium glutamate during the neonatal period. At 3 months old, the Barnes maze assay was performed to evaluate spatial memory function. Microelectrodes were used to measure electrophysiological function in the hippocampal CA1 region. Western blot assay was used to determine expression levels of glutamate ionotropic receptor NMDA type subunit 2A (GluN2A) and GluN2B in the hippocampus. Enzyme-linked immunosorbent assay was used to determine levels of interleukin-1β, tumor necrosis factor α, and interleukin-6 in the hippocampus and cerebral cortex, as well as hippocampal amyloid beta (Aβ)1-40 and Aβ1-42 levels. Our results showed that in the rat model of type 2 diabetes mellitus caused by monosodium glutamate exposure during the neonatal period, latency was prolonged and the number of errors increased in the Barnes maze. Further, latency was increased and time in the escape platform quadrant shortened. Number of times crossing the platform was also reduced in the Morris water maze. After high frequency stimulation of the hippocampus, synaptic transmission was inhibited, expression of GluN2A and GluN2B were decreased in the hippocampus, expression of interleukin 1β, interleukin 6, and tumor necrosis factor α was increased in the hippocampus and cortex, and levels of Aβ1-40 and Aβ1-42 were increased in the hippocampus. These findings confirm that type 2 diabetes mellitus induced by neonatal monosodium glutamate exposure results in Alzheimer-like neuropathological changes and further causes cognitive deficits and neurodegeneration in young adulthood.
Keywords: Alzheimer's disease; cognitive deficits; hyperglycemia; hyperinsulinemia; insulin resistance; monosodium glutamate; neonatal period; nerve regeneration; neural regeneration; type 2 diabetes mellitus.
Conflict of interest statement
All authors declare that no potential or actual conflicts of interest including any financial or personal relationships with other people or research organizations within two years of beginning this work submitted that could inappropriately influence (bias) their work
Figures
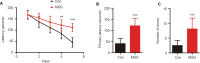
Similar articles
-
Monosodium glutamate exposure during the neonatal period leads to cognitive deficits in adult Sprague-Dawley rats.Neurosci Lett. 2018 Aug 24;682:39-44. doi: 10.1016/j.neulet.2018.06.008. Epub 2018 Jun 6. Neurosci Lett. 2018. PMID: 29885453
-
Intracerebroventricular injection of Aβ1-42 combined with two-vessel occlusion accelerate Alzheimer's disease development in rats.Pathol Res Pract. 2018 Oct;214(10):1583-1595. doi: 10.1016/j.prp.2018.07.020. Epub 2018 Jul 25. Pathol Res Pract. 2018. PMID: 30087036
-
GLP-1 analogue CJC-1131 prevents amyloid β protein-induced impirments of spatial memory and synaptic plasticity in rats.Behav Brain Res. 2017 May 30;326:237-243. doi: 10.1016/j.bbr.2017.03.018. Epub 2017 Mar 15. Behav Brain Res. 2017. PMID: 28315374
-
Can Alzheimer's Disease Be Secondary to Type-2 Diabetes Mellitus?Cureus. 2022 Nov 8;14(11):e31273. doi: 10.7759/cureus.31273. eCollection 2022 Nov. Cureus. 2022. PMID: 36505102 Free PMC article. Review.
-
The Effect of Diabetes Mellitus on Apoptosis in Hippocampus: Cellular and Molecular Aspects.Int J Prev Med. 2016 Mar 10;7:57. doi: 10.4103/2008-7802.178531. eCollection 2016. Int J Prev Med. 2016. PMID: 27076895 Free PMC article. Review.
Cited by
-
Soy isoflavones ameliorate the cognitive dysfunction of Goto-Kakizaki rats by activating the Nrf2-HO-1 signalling pathway.Aging (Albany NY). 2020 Nov 7;12(21):21344-21354. doi: 10.18632/aging.103877. Epub 2020 Nov 7. Aging (Albany NY). 2020. PMID: 33180745 Free PMC article.
-
Hippocampal subfields atrophy contribute more to cognitive impairment in middle-aged patients with type 2 diabetes rather than microvascular lesions.Acta Diabetol. 2021 Aug;58(8):1023-1033. doi: 10.1007/s00592-020-01670-x. Epub 2021 Mar 22. Acta Diabetol. 2021. PMID: 33751221
-
Hypothesis and Theory: Circulating Alzheimer's-Related Biomarkers in Type 2 Diabetes. Insight From the Goto-Kakizaki Rat.Front Neurol. 2019 Jun 26;10:649. doi: 10.3389/fneur.2019.00649. eCollection 2019. Front Neurol. 2019. PMID: 31293498 Free PMC article.
-
N-methyl-D-aspartic acid receptor 2A functionalized stationary phase: A reliable method for pursuing potential ligands against Alzheimer's disease from natural products.CNS Neurosci Ther. 2023 May;29(5):1290-1299. doi: 10.1111/cns.14101. Epub 2023 Jan 27. CNS Neurosci Ther. 2023. PMID: 36708133 Free PMC article.
-
Is L-Glutamate Toxic to Neurons and Thereby Contributes to Neuronal Loss and Neurodegeneration? A Systematic Review.Brain Sci. 2022 Apr 29;12(5):577. doi: 10.3390/brainsci12050577. Brain Sci. 2022. PMID: 35624964 Free PMC article. Review.
References
-
- Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich BL, Finch CE, Frautschy S, Griffin WS, Hampel H, Hull M, Landreth G, Lue L, Mrak R, Mackenzie IR, McGeer PL, et al. Inflammation and Alzheimer's disease. Neurobiol Aging. 2000;21:383–421. - PMC - PubMed
-
- Arendt T. Synaptic degeneration in Alzheimer's disease. Acta Neuropathol. 2009;118:167–179. - PubMed
-
- Bahrmann A, Bahrmann P, Kubiak T, Kopf D, Oster P, Sieber CC, Daniel WG. Diabetes and dementia. Z Gerontol Geriatr. 2012;45:17–22. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

