Durability of initial antidiabetic monotherapy and subsequent treatment adjustment patterns among newly treated type 2 diabetes patients
- PMID: 30233191
- PMCID: PMC6130268
- DOI: 10.2147/TCRM.S169964
Durability of initial antidiabetic monotherapy and subsequent treatment adjustment patterns among newly treated type 2 diabetes patients
Abstract
Background: As newly available antidiabetic drugs (ADs) are used more commonly as initial hypoglycemic choice for early stage diabetes patients, there is an urgent need to investigate how these agents may differ in treatment durability relative to metformin. This study aimed to investigate the incidence and risk of treatment adjustment among newly treated type 2 diabetes mellitus (T2DM) patients receiving an oral AD as initial monotherapy.
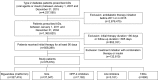
Methods: T2DM patients registered in the National Health Insurance Program who were newly prescribed an oral AD were identified. Time to treatment addition or switch to alternative antidiabetic therapy was determined using the Kaplan-Meier survival analysis. Cox proportional hazards regression was performed to estimate the hazard ratio (HR) after adjusting for potential confounding factors.
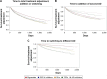
Results: The median time to treatment adjustment was shorter for sulfonylureas (SUs), dipeptidyl peptidase-4 (DPP-4) inhibitors, alphaglucosidase (AG) inhibitors, and thiazolidinediones (TZDs) compared to that for metformin. Initiation of therapy with SUs or DPP-4 inhibitors was associated with a significantly higher risk of both treatment addition and switching than with metformin (HR 1.49 versus 1.47 for overall treatment adjustment, respectively). In contrast, among incident users of AG inhibitors or TZDs, only the hazard of switch was substantially increased compared to metformin starters (6.19, 95% confidence interval [CI] 5.77-6.64 and 7.31, 95% CI 6.35-8.42, respectively). When addition and switch events were collectively assessed, the risk of treatment adjustment was significantly elevated in all non-metformin cohorts.
Conclusion: Our results demonstrated that the durability of metformin as an initial monotherapy was superior to that of other ADs, including newer classes of antidiabetics, and appeared to be more effective in delaying treatment adjustment in real-world clinical practice.
Keywords: antidiabetic drugs; drug utilization patterns; type 2 diabetes.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures
Similar articles
-
Risks of cardiovascular diseases associated with dipeptidyl peptidase-4 inhibitors and other antidiabetic drugs in patients with type 2 diabetes: a nation-wide longitudinal study.Cardiovasc Diabetol. 2016 Mar 1;15:41. doi: 10.1186/s12933-016-0350-4. Cardiovasc Diabetol. 2016. PMID: 26932742 Free PMC article.
-
Important differences in the durability of glycaemic response among second-line treatment options when added to metformin in type 2 diabetes: a retrospective cohort study.Ann Med. 2016;48(4):224-34. doi: 10.3109/07853890.2016.1157263. Epub 2016 Mar 16. Ann Med. 2016. PMID: 26982210
-
Association of Hemoglobin A1c Levels With Use of Sulfonylureas, Dipeptidyl Peptidase 4 Inhibitors, and Thiazolidinediones in Patients With Type 2 Diabetes Treated With Metformin: Analysis From the Observational Health Data Sciences and Informatics Initiative.JAMA Netw Open. 2018 Aug 3;1(4):e181755. doi: 10.1001/jamanetworkopen.2018.1755. JAMA Netw Open. 2018. PMID: 30646124 Free PMC article.
-
Choice of Glucose-Lowering Drugs as Initial Monotherapy for Type 2 Diabetes Patients with Contraindications or Intolerance to Metformin: A Systematic Review and Meta-Analysis.J Clin Med. 2022 Nov 30;11(23):7094. doi: 10.3390/jcm11237094. J Clin Med. 2022. PMID: 36498669 Free PMC article. Review.
-
Dipeptidyl peptidase-4 inhibitors for treatment of type 2 diabetes mellitus in the clinical setting: systematic review and meta-analysis.BMJ. 2012 Mar 12;344:e1369. doi: 10.1136/bmj.e1369. BMJ. 2012. PMID: 22411919
Cited by
-
Effects of early medication treatment and metformin use for cancer prevention in diabetes patients: a nationwide sample cohort study in Korea using extended landmark time analysis.Epidemiol Health. 2021;43:e2021103. doi: 10.4178/epih.e2021103. Epub 2021 Dec 17. Epidemiol Health. 2021. PMID: 34922421 Free PMC article.
-
Impact of metformin on statin-associated myopathy risks in dyslipidemia patients.Pharmacol Res Perspect. 2023 Aug;11(4):e01114. doi: 10.1002/prp2.1114. Pharmacol Res Perspect. 2023. PMID: 37417539 Free PMC article.
-
Incident infection risks depending on oral antidiabetic exposure in insulin-treated type 2 diabetes patients.Sci Rep. 2023 Oct 27;13(1):18462. doi: 10.1038/s41598-023-45793-x. Sci Rep. 2023. PMID: 37891260 Free PMC article.
-
Treatment Patterns of Diabetes in Italy: A Population-Based Study.Front Pharmacol. 2019 Aug 6;10:870. doi: 10.3389/fphar.2019.00870. eCollection 2019. Front Pharmacol. 2019. PMID: 31447672 Free PMC article.
-
Real World Use of Antidiabetic Drugs in the Years 2011-2017: A Population-Based Study from Southern Italy.Int J Environ Res Public Health. 2020 Dec 18;17(24):9514. doi: 10.3390/ijerph17249514. Int J Environ Res Public Health. 2020. PMID: 33353081 Free PMC article.
References
-
- Korean Diabetes Association [webpage on the Internet] Korean Treatment Guideline for Diabetes. 2015. [Accessed January 8, 2018]. Available from: http://www.diabetes.or.kr/pro/publish/guide.php?mode=list.
-
- American Diabetes Association Standards of medical care in diabetes-2017. Diabetes Care. 2017;40(suppl 1):S1–S132. - PubMed
-
- Noh Y, Kang DR, Kim DJ, Lee KJ, Lee S, Shin S. Impact of clinical evidence communications and drug regulation changes concerning rosiglitazone on prescribing patterns of antidiabetic therapies. Pharmacoepidemiol Drug Saf. 2017;26(11):1338–1346. - PubMed
-
- Williams-Herman D, Johnson J, Teng R, et al. Efficacy and safety of sitagliptin and metformin as initial combination therapy and as monotherapy over 2 years in patients with type 2 diabetes. Diabetes Obes Metab. 2010;12(5):442–451. - PubMed
-
- Aschner P, Katzeff HL, Guo H, et al. Sitagliptin Study 049 Group Efficacy and safety of monotherapy of sitagliptin compared with metformin in patients with type 2 diabetes. Diabetes Obes Metab. 2010;12(3):252–261. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

