Altered fractionation radiotherapy with or without chemotherapy in the treatment of head and neck cancer: a network meta-analysis
- PMID: 30233208
- PMCID: PMC6129020
- DOI: 10.2147/OTT.S172018
Altered fractionation radiotherapy with or without chemotherapy in the treatment of head and neck cancer: a network meta-analysis
Abstract
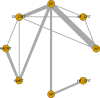
Objectives: A Bayesian network meta-analysis (NMA) was conducted in patients with head and neck cancers (HNCs) to estimate the efficacy and safety of treatment with conventional fractionation radiotherapy (CF), conventional fractionation chemoradiotherapy (CF_CRT), hyperfractionated radiotherapy (HF), hyperfractionated chemoradiotherapy (HF_CRT), accelerated fractionation radiotherapy, accelerated fractionation chemoradiotherapy, accelerated hyperfractionated radiotherapy (HART) or accelerated hyperfractionated chemoradiotherapy (HACRT) to identify superior treatments to aid in clinical decisions.
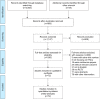
Methods: PubMed, EMBASE and the Cochrane Central Register of Controlled Trials (CENTRAL) were searched for potentially eligible randomized controlled trials up to December 2016. Overall survival (OS), disease-free survival (DFS) and locoregional control (LRC) were considered efficacy outcomes, whereas acute toxicity and late toxicity on skin and mucosa were considered safety outcomes. The surface under the cumulative ranking curve (SUCRA) was calculated to rank each treatment in each index.
Results: Data from 72 trials with 21,868 participants were included in the analysis. Concerning OS, all treatments were associated with a significant advantage compared to CF alone, with HR effect sizes ranging from 0.64 to 0.83, and HACRT was significantly more effective than all the other treatments. The network comparisons of both HACRT vs HART and HF_CRT vs HF demonstrated a higher OS benefit, with an HR of 0.78 (95% credible interval [CrI]: 0.64-0.95) and 0.78 (95% CrI: 0.61-0.99), respectively. The results of SUCRA indicated that HACRT had the best ranking for OS and LRC, HF_CRT for DFS, HART for acute and late skin toxicity, CF_CRT for acute mucosal toxicity and HF_CRT for late mucosal toxicity.
Conclusion: The NMA results support the notion that HACRT is the preferable treatment modality for HNCs because it has better rankings in all three efficacy indexes, although it does present a high risk of acute mucosal toxicity.
Keywords: altered fractionation radiotherapy; head and neck cancer; network meta-analysis; randomized controlled trials.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures


Similar articles
-
Accelerated Hyperfractionated Radiotherapy versus Conventional Fractionation Radiotherapy for Head and Neck Cancer: A Meta-Analysis of Randomized Controlled Trials.J Oncol. 2019 Nov 28;2019:7634746. doi: 10.1155/2019/7634746. eCollection 2019. J Oncol. 2019. PMID: 31885584 Free PMC article.
-
[Controlled clinical trials of hyperfractionated and accelerated radiotherapy in otorhinolaryngologic cancers].Bull Acad Natl Med. 1998;182(6):1247-60; discussion 1261. Bull Acad Natl Med. 1998. PMID: 9812410 Clinical Trial. French.
-
Neoadjuvant treatments for resectable rectal cancer: A network meta-analysis.Exp Ther Med. 2020 Apr;19(4):2604-2614. doi: 10.3892/etm.2020.8494. Epub 2020 Feb 5. Exp Ther Med. 2020. PMID: 32256740 Free PMC article.
-
Altered fractionation schedules in radiotherapy of head and neck cancer. A review.Tumori. 1992 Oct 31;78(5):311-25. doi: 10.1177/030089169207800506. Tumori. 1992. PMID: 1494804 Review.
-
Efficacy and safety of traditional chemotherapies for patients with ovarian neoplasm: a network meta-analysis.Oncotarget. 2017 Mar 30;8(35):59867-59877. doi: 10.18632/oncotarget.16729. eCollection 2017 Aug 29. Oncotarget. 2017. PMID: 28938689 Free PMC article. Review.
Cited by
-
Prospective Study to Compare Efficacy of Conventional Chemoradiotherapy with Hypofractionated Chemoradiotherapy in Locally Advanced Carcinoma of Oropharynx.Asian Pac J Cancer Prev. 2023 Dec 1;24(12):4077-4083. doi: 10.31557/APJCP.2023.24.12.4077. Asian Pac J Cancer Prev. 2023. PMID: 38156840 Free PMC article.
References
-
- Marcial VA, Pajak TF, Chang C, Tupchong L, Stetz J. Hyperfractionated photon radiation therapy in the treatment of advanced squamous cell carcinoma of the oral cavity, pharynx, larynx, and sinuses, using radiation therapy as the only planned modality: (preliminary report) by the Radiation Therapy Oncology Group (RTOG) Int J Radiat Oncol Biol Phys. 1987;13(1):41–47. - PubMed
-
- Sanchiz F, Milla A, Torner J, et al. Single fraction per day versus two fractions per day versus radiochemotherapy in the treatment of head and neck cancer. Int J Radiat Oncol Biol Phys. 1990;19(6):1347–1350. - PubMed
-
- Pinto LHJ, Canary PCV, Araujo CMM, Bacelar SC, Souhami L. Prospective randomized trial comparing hyperfractionated versus conventional radiotherapy in stages III and IV oropharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 1991;21(3):557–562. - PubMed
-
- Horiot JC, Fur R, N’Guyen T, et al. Hyperfractionation versus conventional fractionation in oropharyngeal carcinoma: final analysis of a randomized trial of the EORTC cooperative group of radiotherapy. Radiother Oncol J Eur Soc Ther Radiol Oncol. 1992;25(4):231–241. Available from: http://onlinelibrary.wiley.com/o/cochrane/clcentral/articles/983/CN-0008.... - PubMed
-
- Bogaert W, Schueren E, Horiot JC, et al. The EORTC randomized trial on three fractions per day and misonidazole (trial no. 22811) in advanced head and neck cancer: long-term results and side effects. Radiother Oncol J Eur Soc Ther Radiol Oncol. 1995;35(2):91–99. Available from: http://onlinelibrary.wiley.com/o/cochrane/clcentral/articles/578/CN-0011.... - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

