A History of Drug Discovery for Treatment of Nausea and Vomiting and the Implications for Future Research
- PMID: 30233361
- PMCID: PMC6131675
- DOI: 10.3389/fphar.2018.00913
A History of Drug Discovery for Treatment of Nausea and Vomiting and the Implications for Future Research
Abstract
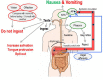
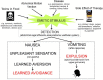
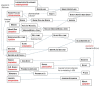
The origins of the major classes of current anti-emetics are examined. Serendipity is a recurrent theme in discovery of their anti-emetic properties and repurposing from one indication to another is a continuing trend. Notably, the discoveries have occurred against a background of company mergers and changing anti-emetic requirements. Major drug classes include: (i) Muscarinic receptor antagonists-originated from historical accounts of plant extracts containing atropine and hyoscine with development stimulated by the need to prevent sea-sickness among soldiers during beach landings; (ii) Histamine receptor antagonists-searching for replacements for the anti-malaria drug quinine, in short supply because of wartime shipping blockade, facilitated the discovery of histamine (H1) antagonists (e.g., dimenhydrinate), followed by serendipitous discovery of anti-emetic activity against motion sickness in a patient undergoing treatment for urticaria; (iii) Phenothiazines and dopamine receptor antagonists-investigations of their pharmacology as "sedatives" (e.g., chlorpromazine) implicated dopamine receptors in emesis, leading to development of selective dopamine (D2) receptor antagonists (e.g., domperidone with poor ability to penetrate the blood-brain barrier) as anti-emetics in chemotherapy and surgery; (iv) Metoclopramide and selective 5-hydroxytryptamine3(5-HT3) receptor antagonists-metoclopramide was initially assumed to act only via D2 receptor antagonism but subsequently its gastric motility stimulant effect (proposed to contribute to the anti-emetic action) was shown to be due to 5-hydroxytryptamine4 receptor agonism. Pre-clinical studies showed that anti-emetic efficacy against the newly-introduced, highly emetic, chemotherapeutic agent cisplatin was due to antagonism at 5-HT3 receptors. The latter led to identification of selective 5-HT3 receptor antagonists (e.g., granisetron), a major breakthrough in treatment of chemotherapy-induced emesis; (v) Neurokinin1receptor antagonists-antagonists of the actions of substance P were developed as analgesics but pre-clinical studies identified broad-spectrum anti-emetic effects; clinical studies showed particular efficacy in the delayed phase of chemotherapy-induced emesis. Finally, the repurposing of different drugs for treatment of nausea and vomiting is examined, particularly during palliative care, and also the challenges in identifying novel anti-emetic drugs, particularly for treatment of nausea as compared to vomiting. We consider the lessons from the past for the future and ask why there has not been a major breakthrough in the last 20 years.
Keywords: 5-hydroxytryptamine3 receptor antagonists; drug discovery; histamine H1 receptor antagonists; metoclopramide; muscarinic receptor antagonists; nausea and vomiting; neurokinin1 receptor antagonists; olanzapine.
Figures




References
-
- Aapro M., Rugo H., Rossi G., Rizzi G., Borroni M. E., Bondarenko I., et al. . (2014). A randomized phase III study evaluating the efficacy and safety of NEPA, a fixed-dose combination of netupitant and palonosetron, for prevention of chemotherapy-induced nausea and vomiting following moderately emetogenic chemotherapy. Ann. Oncol. 25, 1328–1333. 10.1093/annonc/mdu101 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

