An Insect Prostaglandin E2 Synthase Acts in Immunity and Reproduction
- PMID: 30233407
- PMCID: PMC6131586
- DOI: 10.3389/fphys.2018.01231
An Insect Prostaglandin E2 Synthase Acts in Immunity and Reproduction
Abstract
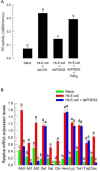
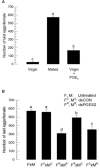
Eicosanoids, oxygenated metabolites of C20 polyunsaturated fatty acids (PUFAs), mediate fundamental physiological processes, including immune reactions and reproduction, in insects. Prostaglandins (PGs) make up one group of eicosanoids, of which PGE2 is a relatively well-known mediator in various insect taxa. While PG biosynthesis has been reported, the specific biosynthetic pathway for PGE2 is not known in insects. Here, we posed the hypothesis that Se-mPGES2 mediates biosynthesis of physiologically active PGE2 through its cognate protein. To test this hypothesis, we interrogated a transcriptome of the lepidopteran insect, Spodoptera exigua, to identify a candidate PGE2 synthase (Se-mPGES2) and analyzed its sequence and expression. Its predicted amino acid sequence contains a consensus thioredoxin homology sequence (Cys-x-x-Cys) responsible for catalytic activity along with an N-terminal membrane-associated hydrophobic domain and C-terminal cytosolic domain. It also shares sequence homology (36.5%) and shares almost overlapping three dimensional structures with a membrane-bound human PGES2 (mPGES2). Se-mPGES2 was expressed in all developmental stages with high peaks during the late larval instar and adult stages. Immune challenge significantly up-regulated its expression levels in hemocytes and fat body. Injecting double-stranded RNA (dsRNA) specific to Se-mPGES2 significantly impaired two cellular immune responses, hemocyte-spreading behavior and nodule formation following bacterial challenge. Humoral immunity was also significantly suppressed, registered as reduced phenoloxidase activity and antimicrobial peptide expression levels. The suppressed immune responses were reversed following PGE2, but not arachidonic acid (AA), treatments. RNAi treatments also reduced the egg-laying behavior of females. Control females mated with the RNAi-treated males led to substantially reduced egg-laying behavior, which was also reversed following PGE2 injections into females. These results strongly bolster our hypothesis that Se-mPGES2 acts in the biosynthesis of PGE2, a crucial biochemical signal mediating immune and reproductive physiology of S. exigua.
Keywords: PGE2; PGES; Spodoptera exigua; eicosanoids; immunity; reproduction.
Figures
Similar articles
-
Eicosanoid Signaling in Insect Immunology: New Genes and Unresolved Issues.Genes (Basel). 2021 Feb 1;12(2):211. doi: 10.3390/genes12020211. Genes (Basel). 2021. PMID: 33535438 Free PMC article. Review.
-
The first report of prostacyclin and its physiological roles in insects.Gen Comp Endocrinol. 2021 Jan 15;301:113659. doi: 10.1016/j.ygcen.2020.113659. Epub 2020 Nov 7. Gen Comp Endocrinol. 2021. PMID: 33166533
-
Biosynthetic pathway of arachidonic acid in Spodoptera exigua in response to bacterial challenge.Insect Biochem Mol Biol. 2019 Aug;111:103179. doi: 10.1016/j.ibmb.2019.103179. Epub 2019 Jun 28. Insect Biochem Mol Biol. 2019. PMID: 31255640
-
PGE2 mediates cytoskeletal rearrangement of hemocytes via Cdc42, a small G protein, to activate actin-remodeling factors in Spodoptera exigua (Lepidoptera: Noctuidae).Arch Insect Biochem Physiol. 2019 Dec;102(4):e21607. doi: 10.1002/arch.21607. Epub 2019 Jul 23. Arch Insect Biochem Physiol. 2019. PMID: 31338878
-
Prostaglandins and Other Eicosanoids in Insects: Biosynthesis and Biological Actions.Front Physiol. 2019 Feb 7;9:1927. doi: 10.3389/fphys.2018.01927. eCollection 2018. Front Physiol. 2019. PMID: 30792667 Free PMC article. Review.
Cited by
-
Prostaglandins regulate humoral immune responses in Aedes aegypti.PLoS Negl Trop Dis. 2020 Oct 23;14(10):e0008706. doi: 10.1371/journal.pntd.0008706. eCollection 2020 Oct. PLoS Negl Trop Dis. 2020. PMID: 33095767 Free PMC article.
-
Thromboxane Mobilizes Insect Blood Cells to Infection Foci.Front Immunol. 2021 Dec 20;12:791319. doi: 10.3389/fimmu.2021.791319. eCollection 2021. Front Immunol. 2021. PMID: 34987515 Free PMC article.
-
Eicosanoid Signaling in Insect Immunology: New Genes and Unresolved Issues.Genes (Basel). 2021 Feb 1;12(2):211. doi: 10.3390/genes12020211. Genes (Basel). 2021. PMID: 33535438 Free PMC article. Review.
-
Functional characterization of eicosanoid signaling in Drosophila development.PLoS Genet. 2025 May 9;21(5):e1011705. doi: 10.1371/journal.pgen.1011705. eCollection 2025 May. PLoS Genet. 2025. PMID: 40344083 Free PMC article.
-
Differential gene expression in response to fungal pathogen exposure in the aquatic invertebrate, Daphnia dentifera.Ecol Evol. 2023 Jul 28;13(8):e10354. doi: 10.1002/ece3.10354. eCollection 2023 Aug. Ecol Evol. 2023. PMID: 37529587 Free PMC article.
References
-
- Bichon A., Boukhatem N., Gay P., Dru P., Terzian H., Petitjean A. M., et al. (2001). Genetic and molecular features of Su(P), a gene that interacts with ref(2)P in male fertility of Drosophila melanogaster. Mol. Genet. Genomics 265 354–361. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

