The Peripheral Stalk of Rotary ATPases
- PMID: 30233414
- PMCID: PMC6131620
- DOI: 10.3389/fphys.2018.01243
The Peripheral Stalk of Rotary ATPases
Abstract
Rotary ATPases are a family of enzymes that are thought of as molecular nanomotors and are classified in three types: F, A, and V-type ATPases. Two members (F and A-type) can synthesize and hydrolyze ATP, depending on the energetic needs of the cell, while the V-type enzyme exhibits only a hydrolytic activity. The overall architecture of all these enzymes is conserved and three main sectors are distinguished: a catalytic core, a rotor and a stator or peripheral stalk. The peripheral stalks of the A and V-types are highly conserved in both structure and function, however, the F-type peripheral stalks have divergent structures. Furthermore, the peripheral stalk has other roles beyond its stator function, as evidenced by several biochemical and recent structural studies. This review describes the information regarding the organization of the peripheral stalk components of F, A, and V-ATPases, highlighting the key differences between the studied enzymes, as well as the different processes in which the structure is involved.
Keywords: ATP synthase; archaea; bacteria; chloroplast; coiled-coils; mitochondria; peripheral stalk.
Figures

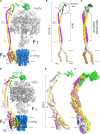


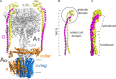
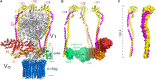


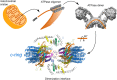
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

