Circular and Leaderless Bacteriocins: Biosynthesis, Mode of Action, Applications, and Prospects
- PMID: 30233551
- PMCID: PMC6131525
- DOI: 10.3389/fmicb.2018.02085
Circular and Leaderless Bacteriocins: Biosynthesis, Mode of Action, Applications, and Prospects
Abstract
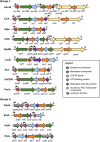
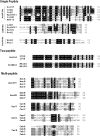
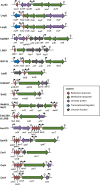
Bacteriocins are a huge family of ribosomally synthesized peptides known to exhibit a range of bioactivities, most predominantly antibacterial activities. Bacteriocins from lactic acid bacteria are of particular interest due to the latter's association to food fermentation and the general notion of them to be safe. Among the family of bacteriocins, the groups known as circular bacteriocins and leaderless bacteriocins are gaining more attention due to their enormous potential for industrial application. Circular bacteriocins and leaderless bacteriocins, arguably the least understood groups of bacteriocins, possess distinctively peculiar characteristics in their structures and biosynthetic mechanisms. Circular bacteriocins have N-to-C- terminal covalent linkage forming a structurally distinct circular peptide backbone. The circular nature of their structures provides them superior stability against various stresses compared to most linear bacteriocins. The molecular mechanism of their biosynthesis, albeit has remained poorly understood, is believed to possesses huge application prospect as it can serve as scaffold in bioengineering other biologically important peptides. On the other hand, while most bacteriocins are synthesized as inactive precursor peptides, which possess an N-terminal leader peptide attached to a C-terminal propeptide, leaderless bacteriocins are atypical as they do not have an N-terminal leader peptide, hence the name. Leaderless bacteriocins are active right after translation as they do not undergo any post-translational processing common to other groups of bacteriocins. This "simplicity" in the biosynthesis of leaderless bacteriocins offers a huge commercial potential as scale-up production systems are considerably easier to assemble. In this review, we summarize the current studies of both circular and leaderless bacteriocins, highlighting the progress in understanding their biosynthesis, mode of action, application and their prospects.
Keywords: bacteriocin biosynthesis; bacteriocins; circular bacteriocins; lactic acid bacteria; leaderless bacteriocins; mode of action.
Figures



References
-
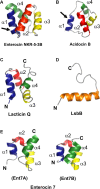
- Acedo J. Z., Van Belkum M. J., Lohans C. T., Towle K. M., Miskolzie M., Vederas J. C. (2016). Nuclear magnetic resonance solution structures of lacticin Q and aureocin A53 reveal a structural motif conserved among leaderless bacteriocins with broad-spectrum activity. Biochemistry 55 733–742. 10.1021/acs.biochem.5b01306 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

