The Role of Collectins and Galectins in Lung Innate Immune Defense
- PMID: 30233589
- PMCID: PMC6131309
- DOI: 10.3389/fimmu.2018.01998
The Role of Collectins and Galectins in Lung Innate Immune Defense
Abstract
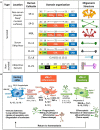
Different families of endogenous lectins use complementary defense strategies against pathogens. They may recognize non-self glycans typically found on pathogens and/or host glycans. The collectin and galectin families are prominent examples of these two lectin categories. Collectins are C-type lectins that contain a carbohydrate recognition domain and a collagen-like domain. Members of this group include surfactant protein A (SP-A) and D (SP-D), secreted by the alveolar epithelium to the alveolar fluid. Lung collectins bind to several microorganisms, which results in pathogen aggregation and/or killing, and enhances phagocytosis of pathogens by alveolar macrophages. Moreover, SP-A and SP-D influence macrophage responses, contributing to resolution of inflammation, and SP-A is essential for tissue-repair functions of macrophages. Galectins also function by interacting directly with pathogens or by modulating the immune system in response to the infection. Direct binding may result in enhanced or impaired infection of target cells, or can have microbicidal effects. Immunomodulatory effects of galectins include recruitment of immune cells to the site of infection, promotion of neutrophil function, and stimulation of the bactericidal activity of infected macrophages. Moreover, intracellular galectins can serve as danger receptors, promoting autophagy of the invading pathogen. This review will focus on the role of collectins and galectins in pathogen clearance and immune response activation in infectious diseases of the respiratory system.
Keywords: alternatively activated macrophages; autophagy; infection; inflammation; lung homeostasis; respiratory pathogens; surfactant proteins; tissue repair.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

