Thymosin β4 promotes glucose-impaired endothelial progenitor cell function via Akt/endothelial nitric oxide synthesis signaling pathway
- PMID: 30233693
- PMCID: PMC6143828
- DOI: 10.3892/etm.2018.6593
Thymosin β4 promotes glucose-impaired endothelial progenitor cell function via Akt/endothelial nitric oxide synthesis signaling pathway
Abstract
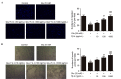
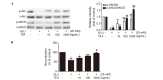
Circulating endothelial progenitor cells (EPCs) are a subtype of hematopoietic stem cells, which can differentiate into endothelial cells and restore endothelial function. However, high glucose decreases the number and impairs the function of EPCs. A previous study showed that thymosin β4 (Tβ4), a pleiotropic peptide beneficial for multiple functions of various types of cells, could promote EPC migration and dose-dependently upregulate the phosphorylation of Akt and endothelial nitric oxide synthesis signaling (eNOS). In present study, the hypothesis that Tβ4 can improve glucose-suppressed EPC functions via the Akt/eNOS signaling pathway and restores the production of nitric oxide (NO) is investigated. EPCs were isolated from the peripheral blood of healthy volunteers and formed a cobblestone shape after 3-4 weeks of cultivation. Then, EPCs were treated with high concentrations of glucose (25 mM) for 4 days and administrated with Tβ4 for further study. Transwell migration and tube formation assays were performed to access the migratory and angiogenic ability of EPCs. In addition, the quantity of Akt, eNOS and the concentration of nitric oxide (NO) was investigated. Functional studies showed that high concentrations of glucose significantly suppressed EPC function, while this adverse effect was reversed by the administration of Tβ4. In addition, Akt small interfering (si)RNA and eNOS siRNA were demonstrated to reduce the protective effect of Tβ4 against glucose-impaired EPC functions. These findings suggest that Tβ4 improves glucose-impaired EPC functions via the Akt/eNOS signaling pathway.
Keywords: angiogenesis; endothelial progenitor cells; hyperglycemia; migration; thymosin β4.
Figures

Similar articles
-
Thymosin β4 promotes endothelial progenitor cell angiogenesis via a vascular endothelial growth factor‑dependent mechanism.Mol Med Rep. 2018 Aug;18(2):2314-2320. doi: 10.3892/mmr.2018.9199. Epub 2018 Jun 20. Mol Med Rep. 2018. PMID: 29956769
-
Globular adiponectin improves high glucose-suppressed endothelial progenitor cell function through endothelial nitric oxide synthase dependent mechanisms.J Mol Cell Cardiol. 2011 Jul;51(1):109-19. doi: 10.1016/j.yjmcc.2011.03.008. Epub 2011 Mar 31. J Mol Cell Cardiol. 2011. PMID: 21439968
-
Thymosin β4 reduces senescence of endothelial progenitor cells via the PI3K/Akt/eNOS signal transduction pathway.Mol Med Rep. 2013 Feb;7(2):598-602. doi: 10.3892/mmr.2012.1180. Epub 2012 Nov 12. Mol Med Rep. 2013. PMID: 23151623
-
Risk factors for coronary artery disease, circulating endothelial progenitor cells, and the role of HMG-CoA reductase inhibitors.Kidney Int. 2005 May;67(5):1672-6. doi: 10.1111/j.1523-1755.2005.00261.x. Kidney Int. 2005. PMID: 15840010 Review.
-
Current perspective of pathophysiological and interventional effects on endothelial progenitor cell biology: focus on PI3K/AKT/eNOS pathway.Int J Cardiol. 2010 Oct 29;144(3):350-66. doi: 10.1016/j.ijcard.2010.04.018. Epub 2010 May 4. Int J Cardiol. 2010. PMID: 20444511 Review.
Cited by
-
[Research advances on thymosin β4 in promoting wound healing].Zhonghua Shao Shang Yu Chuang Mian Xiu Fu Za Zhi. 2022 Apr 20;38(4):378-384. doi: 10.3760/cma.j.cn501120-20210221-00059. Zhonghua Shao Shang Yu Chuang Mian Xiu Fu Za Zhi. 2022. PMID: 35462518 Free PMC article. Review. Chinese.
References
-
- Fujiyama S, Amano K, Uehira K, Yoshida M, Nishiwaki Y, Nozawa Y, Jin D, Takai S, Miyazaki M, Egashira K, et al. Bone marrow monocyte lineage cells adhere on injured endothelium in a monocyte chemoattractant protein-1-dependent manner and accelerate reendothelialization as endothelial progenitor cells. Circ Res. 2003;93:980–989. doi: 10.1161/01.RES.0000099245.08637.CE. - DOI - PubMed
-
- Werner N, Priller J, Laufs U, Endres M, Böhm M, Dirnagl U, Nickenig G. Bone marrow-derived progenitor cells modulate vascular reendothelialization and neointimal formation: Effect of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibition. Arterioscler Thromb Vasc Biol. 2002;22:1567–1572. doi: 10.1161/01.ATV.0000036417.43987.D8. - DOI - PubMed
-
- Loomans CJ, de Koning EJ, Staal FJ, Rookmaaker MB, Verseyden C, de Boer HC, Verhaar MC, Braam B, Rabelink TJ, van Zonneveld AJ. Endothelial progenitor cell dysfunction: A novel concept in the pathogenesis of vascular complications of type 1 diabetes. Diabetes. 2004;53:195–199. doi: 10.2337/diabetes.53.1.195. - DOI - PubMed
-
- Fadini GP, Miorin M, Facco M, Bonamico S, Baesso I, Grego F, Menegolo M, de Kreutzenberg SV, Tiengo A, Agostini C, Avogaro A. Circulating endothelial progenitor cells are reduced in peripheral vascular complications of type 2 diabetes mellitus. J Am Coll Cardiol. 2005;45:1449–1457. doi: 10.1016/j.jacc.2004.11.067. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
