SupplAzithromycin for Prevention of Graft-Versus-Host Disease: A Randomized Placebo-Controlled Trial
- PMID: 30233767
- PMCID: PMC6141429
SupplAzithromycin for Prevention of Graft-Versus-Host Disease: A Randomized Placebo-Controlled Trial
Abstract
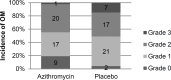
Background: Allogeneic hematopoietic stem cell transplantation has been used widely to treat various types of malignant and non-malignant disorders. Graft-versus-host disease is one of the main complications of this procedure which is associated with considerable mortality and affects quality of life. Despite careful selection of HLA-matched donors and implementing immunosuppressive therapy, the incidence rate of graft-versus-host disease remains high. Macrolide antibiotics are well-known immunomodulatory agents and have been effective as prophylaxis for graft-versus-host disease in preclinical studies. Materials and Methods: Ninety-six adult patients with acute leukemia were recruited into a double-blind, randomized, placebo-controlled trial. All patients were first-time transplant candidates for a full-matched related or unrelated donor. Patients were allocated to receive azithromycin 500 mg daily (n=48) or placebo (n=48) from day -6 to +12. All patients received high-dose chemotherapy, standard immunosuppressive regimen and supportive care according to institutional protocols. Results: The incidence of acute graft-versus-host disease grade III-IV and chronic graft-versus-host disease garde I-III was not significantly different between the two study arms. Oral mucositis grade 1-3 occurred in significantly lower number of patients in the azithromycin group compared with placebo. Conclusion: Based on the results of this study, protective effect of azithromycin on graft-versus-host disease could not be demonstrated.
Keywords: Azithromycin; Graft-versus-host disease; Mucositis.
Figures
References
-
- Marks DI, Alonso L, Radia R. Allogeneic hematopoietic cell transplantation in adult patients with acute lymphoblastic leukemia. Hematol Oncol Clin North Am. 2014;28(6):995–1009. - PubMed
-
- Cooke KR, Olkiewicz K, Erickson N, et al. The role of endotoxin and the innate immune response in the pathophysiology of acute graft versus host disease. J Endotoxin Res. 2002;8(6):441–8. - PubMed
-
- Ball LM, Egeler RM, et al. l Acute GvHD: pathogenesis and classification. Bone Marrow Transplant. 2008;41(Suppl 2):S58–64. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials