Phenotypic Basis for Matrix Stiffness-Dependent Chemoresistance of Breast Cancer Cells to Doxorubicin
- PMID: 30234012
- PMCID: PMC6134055
- DOI: 10.3389/fonc.2018.00337
Phenotypic Basis for Matrix Stiffness-Dependent Chemoresistance of Breast Cancer Cells to Doxorubicin
Abstract
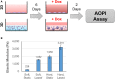
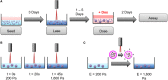
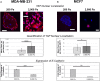
The persistence of drug resistant cell populations following chemotherapeutic treatment is a significant challenge in the clinical management of cancer. Resistant subpopulations arise via both cell intrinsic and extrinsic mechanisms. Extrinsic factors in the microenvironment, including neighboring cells, glycosaminoglycans, and fibrous proteins impact therapy response. Elevated levels of extracellular fibrous proteins are associated with tumor progression and cause the surrounding tissue to stiffen through changes in structure and composition of the extracellular matrix (ECM). We sought to determine how this progressively stiffening microenvironment affects the sensitivity of breast cancer cells to chemotherapeutic treatment. MDA-MB-231 triple negative breast carcinoma cells cultured in a 3D alginate-based hydrogel system displayed a stiffness-dependent response to the chemotherapeutic doxorubicin. MCF7 breast carcinoma cells cultured in the same conditions did not exhibit this stiffness-dependent resistance to the drug. This differential therapeutic response was coordinated with nuclear translocation of YAP, a marker of mesenchymal differentiation. The stiffness-dependent response was lost when cells were transferred from 3D to monolayer cultures, suggesting that endpoint ECM conditions largely govern the response to doxorubicin. To further examine this response, we utilized a platform capable of dynamic ECM stiffness modulation to allow for a change in matrix stiffness over time. We found that MDA-MB-231 cells have a stiffness-dependent resistance to doxorubicin and that duration of exposure to ECM stiffness is sufficient to modulate this response. These results indicate the need for additional tools to integrate mechanical stiffness with therapeutic response and inform decisions for more effective use of chemotherapeutics in the clinic.
Keywords: 3D cell culture; chemotherapy; extracellular matrix; resistance; tumor microenvironment.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

