The Human SLC1A5 (ASCT2) Amino Acid Transporter: From Function to Structure and Role in Cell Biology
- PMID: 30234109
- PMCID: PMC6131531
- DOI: 10.3389/fcell.2018.00096
The Human SLC1A5 (ASCT2) Amino Acid Transporter: From Function to Structure and Role in Cell Biology
Abstract
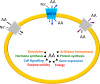
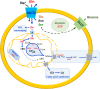
SLC1A5, known as ASCT2, is a neutral amino acid transporter belonging to the SLC1 family and localized in the plasma membrane of several body districts. ASCT2 is an acronym standing for Alanine, Serine, Cysteine Transporter 2 even if the preferred substrate is the conditionally essential amino acid glutamine, with cysteine being a modulator and not a substrate. The studies around amino acid transport in cells and tissues began in the '60s by using radiolabeled compounds and competition assays. After identification of murine and human genes, the function of the coded protein has been studied in cell system and in proteoliposomes revealing that this transporter is a Na+ dependent antiporter of neutral amino acids, some of which are only inwardly transported and others are bi-directionally exchanged. The functional asymmetry merged with the kinetic asymmetry in line with the physiological role of amino acid pool harmonization. An intriguing function has been described for ASCT2 that is exploited as a receptor by a group of retroviruses to infect human cells. Interactions with scaffold proteins and post-translational modifications regulate ASCT2 stability, trafficking and transport activity. Two asparagine residues, namely N163 and N212, are the sites of glycosylation that is responsible for the definitive localization into the plasma membrane. ASCT2 expression increases in highly proliferative cells such as inflammatory and stem cells to fulfill the augmented glutamine demand. Interestingly, for the same reason, the expression of ASCT2 is greatly enhanced in many human cancers. This finding has generated interest in its candidacy as a pharmacological target for new anticancer drugs. The recently solved 3D structure of ASCT2 will aid in the rational design of such therapeutic compounds.
Keywords: ASCT2; SLC1 family; drug design; glutamine; molecular docking; proteoliposomes.
Figures




References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

