A Perfusion Bioreactor System for Cell Seeding and Oxygen-Controlled Cultivation of Three-Dimensional Cell Cultures
- PMID: 30234443
- PMCID: PMC6208160
- DOI: 10.1089/ten.TEC.2018.0204
A Perfusion Bioreactor System for Cell Seeding and Oxygen-Controlled Cultivation of Three-Dimensional Cell Cultures
Abstract
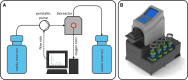
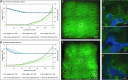
Bioreactor systems facilitate three-dimensional (3D) cell culture by coping with limitations of static cultivation techniques. To allow for the investigation of proper cultivation conditions and the reproducible generation of tissue-engineered grafts, a bioreactor system, which comprises the control of crucial cultivation parameters in independent-operating parallel bioreactors, is beneficial. Furthermore, the use of a bioreactor as an automated cell seeding tool enables even cell distributions on stable scaffolds. In this study, we developed a perfusion microbioreactor system, which enables the cultivation of 3D cell cultures in an oxygen-controlled environment in up to four independent-operating bioreactors. Therefore, perfusion microbioreactors were designed with the help of computer-aided design, and manufactured using the 3D printing technologies stereolithography and fused deposition modeling. A uniform flow distribution in the microbioreactor was shown using a computational fluid dynamics model. For oxygen measurements, microsensors were integrated in the bioreactors to measure the oxygen concentration (OC) in the geometric center of the 3D cell cultures. To control the OC in each bioreactor independently, an automated feedback loop was developed, which adjusts the perfusion velocity according to the oxygen sensor signal. Furthermore, an automated cell seeding protocol was implemented to facilitate the even distribution of cells within a stable scaffold in a reproducible way. As proof of concept, the human mesenchymal stem cell line SCP-1 was seeded on bovine cancellous bone matrix of 1 cm3 and cultivated in the developed microbioreactor system at different oxygen levels. The oxygen control was capable to maintain preset oxygen levels ±0.5% over a cultivation period of several days. Using the automated cell seeding procedure resulted in evenly distributed cells within a stable scaffold. In summary, the developed microbioreactor system enables the cultivation of 3D cell cultures in an automated and thus reproducible way by providing up to four independently operating, oxygen-controlled bioreactors. In combination with the automated cell seeding procedure, the bioreactor system opens up new possibilities to conduct more reproducible experiments to investigate optimal cultivation parameters and to generate tissue-engineering grafts in an oxygen-controlled environment.
Keywords: 3D cell culture; cell seeding; feedback control; oxygen measurement; perfusion microbioreactor.
Conflict of interest statement
No competing financial interests exist.
Figures



Similar articles
-
An axial distribution of seeding, proliferation, and osteogenic differentiation of MC3T3-E1 cells and rat bone marrow-derived mesenchymal stem cells across a 3D Thai silk fibroin/gelatin/hydroxyapatite scaffold in a perfusion bioreactor.Mater Sci Eng C Mater Biol Appl. 2016 Jan 1;58:960-70. doi: 10.1016/j.msec.2015.09.034. Epub 2015 Sep 12. Mater Sci Eng C Mater Biol Appl. 2016. PMID: 26478392
-
Osteogenic Activity on NaOH-Etched Three-Dimensional-Printed Poly-ɛ-Caprolactone Scaffolds in Perfusion or Spinner Flask Bioreactor.Tissue Eng Part C Methods. 2023 Jun;29(6):230-241. doi: 10.1089/ten.tec.2023.0062. Epub 2023 May 30. Tissue Eng Part C Methods. 2023. PMID: 37253166
-
Uniform tissues engineered by seeding and culturing cells in 3D scaffolds under perfusion at defined oxygen tensions.Biorheology. 2006;43(3,4):481-8. Biorheology. 2006. PMID: 16912419
-
The role of perfusion bioreactors in bone tissue engineering.Biomatter. 2012 Oct-Dec;2(4):167-75. doi: 10.4161/biom.22170. Biomatter. 2012. PMID: 23507883 Free PMC article. Review.
-
Spaceflight bioreactor studies of cells and tissues.Adv Space Biol Med. 2002;8:177-95. doi: 10.1016/s1569-2574(02)08019-x. Adv Space Biol Med. 2002. PMID: 12951697 Review.
Cited by
-
Optimization of Oxygen Delivery Within Hydrogels.J Biomech Eng. 2021 Oct 1;143(10):101004. doi: 10.1115/1.4051119. J Biomech Eng. 2021. PMID: 33973004 Free PMC article. Review.
-
Recent advances of natural biopolymeric culture scaffold: synthesis and modification.Bioengineered. 2022 Feb;13(2):2226-2247. doi: 10.1080/21655979.2021.2024322. Bioengineered. 2022. PMID: 35030968 Free PMC article. Review.
-
Closer to Nature: The Role of MSCs in Recreating the Microenvironment of the Hematopoietic Stem Cell Niche in vitro.Transfus Med Hemother. 2022 Jan 14;49(4):258-267. doi: 10.1159/000520932. eCollection 2022 Aug. Transfus Med Hemother. 2022. PMID: 36159960 Free PMC article. Review.
-
3D In Vitro Platform for Cell and Explant Culture in Liquid-like Solids.Cells. 2022 Mar 11;11(6):967. doi: 10.3390/cells11060967. Cells. 2022. PMID: 35326418 Free PMC article.
-
Advances in Engineering Human Tissue Models.Front Bioeng Biotechnol. 2021 Jan 28;8:620962. doi: 10.3389/fbioe.2020.620962. eCollection 2020. Front Bioeng Biotechnol. 2021. PMID: 33585419 Free PMC article.
References
-
- Drosse I., Volkmer E., Capanna R., De Biase P., Mutschler W., and Schieker M. Tissue engineering for bone defect healing: an update on a multi-component approach. Injury 39, S9, 2008 - PubMed
-
- Carvalho J.L., de Carvalho P.H., and de Goes A.M. Advances in biomaterials science and biomaterials applications. In: Pignatello R., ed. Innovative Strategies for Tissue Engineering. London: InTECH Open Limited, 2013, pp. 295–313
-
- Ravichandran A., Liu Y., and Teoh S.H. Review: bioreactor design toward generation of relevant engineered tissues: focus on clinical translation. J Tissue Eng Regen Med 12, e7, 2018 - PubMed
-
- Kasper G., van Griensven M., and Pörtner R. Bioreactor Systems for Tissue Engineering. Berlin, Germany: Springer, 2009
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
