UBE2G1 governs the destruction of cereblon neomorphic substrates
- PMID: 30234487
- PMCID: PMC6185104
- DOI: 10.7554/eLife.40958
UBE2G1 governs the destruction of cereblon neomorphic substrates
Abstract
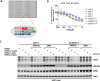
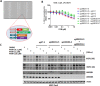
The cereblon modulating agents (CMs) including lenalidomide, pomalidomide and CC-220 repurpose the Cul4-RBX1-DDB1-CRBN (CRL4CRBN) E3 ubiquitin ligase complex to induce the degradation of specific neomorphic substrates via polyubiquitination in conjunction with E2 ubiquitin-conjugating enzymes, which have until now remained elusive. Here we show that the ubiquitin-conjugating enzymes UBE2G1 and UBE2D3 cooperatively promote the K48-linked polyubiquitination of CRL4CRBN neomorphic substrates via a sequential ubiquitination mechanism. Blockade of UBE2G1 diminishes the ubiquitination and degradation of neomorphic substrates, and consequent antitumor activities elicited by all tested CMs. For example, UBE2G1 inactivation significantly attenuated the degradation of myeloma survival factors IKZF1 and IKZF3 induced by lenalidomide and pomalidomide, hence conferring drug resistance. UBE2G1-deficient myeloma cells, however, remained sensitive to a more potent IKZF1/3 degrader CC-220. Collectively, it will be of fundamental interest to explore if loss of UBE2G1 activity is linked to clinical resistance to drugs that hijack the CRL4CRBN to eliminate disease-driving proteins.
Keywords: PROTAC; biochemistry; cancer biology; cereblon; cereblon modulating agents; chemical biology; human; immunomodulatory drugs; ubiquitination.
© 2018, Lu et al.
Conflict of interest statement
GL Employee of Celgene, SW Stephanie Weng: Employee of Celgene, MM Mary Matyskiela: Employee of Celgene, XZ Xinde Zheng: Employee of Celgene, WF Wei Fang: Employee of Celgene, SW Scott Wood: Employee of Celgene, CS Christine Surka: Employee of Celgene, RM Reina Mizukoshi: Employee of Celgene, CL Chin-Chun Lu: Employee of Celgene, DM Derek Mendy: Employee of Celgene, IJ In Sock Jang: Employee of Celgene, KW Kai Wang: Employee of Celgene, MM Mathieu Marella: Employee of Celgene, SC Suzana Couto: Employee of Celgene, BC Brian Cathers: Employee of Celgene, JC James Carmichael: Employee of Celgene, PC Philip Chamberlain: Employee of Celgene, MR Mark Rolfe: Employee of Celgene
Figures















References
-
- Chamberlain PP, Lopez-Girona A, Miller K, Carmel G, Pagarigan B, Chie-Leon B, Rychak E, Corral LG, Ren YJ, Wang M, Riley M, Delker SL, Ito T, Ando H, Mori T, Hirano Y, Handa H, Hakoshima T, Daniel TO, Cathers BE. Structure of the human Cereblon-DDB1-lenalidomide complex reveals basis for responsiveness to thalidomide analogs. Nature Structural & Molecular Biology. 2014;21:803–809. doi: 10.1038/nsmb.2874. - DOI - PubMed
-
- Choi YS, Lee YJ, Lee SY, Shi L, Ha JH, Cheong HK, Cheong C, Cohen RE, Ryu KS. Differential ubiquitin binding by the acidic loops of Ube2g1 and Ube2r1 enzymes distinguishes their Lys-48-ubiquitylation activities. Journal of Biological Chemistry. 2015;290:2251–2263. doi: 10.1074/jbc.M114.624809. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

