Epilepsy-on-a-Chip System for Antiepileptic Drug Discovery
- PMID: 30235116
- PMCID: PMC6585967
- DOI: 10.1109/TBME.2018.2871415
Epilepsy-on-a-Chip System for Antiepileptic Drug Discovery
Abstract
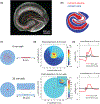
Objective: Hippocampal slice cultures spontaneously develop chronic epilepsy several days after slicing and are used as an in vitro model of post-traumatic epilepsy. Here, we describe a hybrid microfluidic-microelectrode array (μflow-MEA) technology that incorporates a microfluidic perfusion network and electrodes into a miniaturized device for hippocampal slice culture based antiepileptic drug discovery.
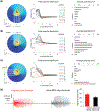
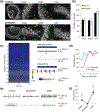
Methods: Field potential simulation was conducted to help optimize the electrode design to detect a seizure-like population activity. Epilepsy-on-a-chip model was validated by chronic electrical recording, neuronal survival quantification, and anticonvulsant test. To demonstrate the application of μflow-MEA in drug discovery, we utilized a two-stage screening platform to identify potential targets for antiepileptic drugs. In Stage I, lactate and lactate dehydrogenase biomarker assays were performed to identify potential drug candidates. In Stage II, candidate compounds were retested with μflow-MEA-based chronic electrical assay to provide electrophysiological confirmation of biomarker results.
Results and conclusion: We screened 12 receptor tyrosine kinases inhibitors, and EGFR/ErbB-2 and cFMS inhibitors were identified as novel antiepileptic compounds.
Significance: This epilepsy-on-a-chip system provides the means for rapid dissection of complex signaling pathways in epileptogenesis, paving the way for high-throughput antiepileptic drug discovery.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

