Propionate-producing bacteria in the intestine may associate with skewed responses of IL10-producing regulatory T cells in patients with relapsing polychondritis
- PMID: 30235279
- PMCID: PMC6147427
- DOI: 10.1371/journal.pone.0203657
Propionate-producing bacteria in the intestine may associate with skewed responses of IL10-producing regulatory T cells in patients with relapsing polychondritis
Abstract
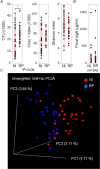
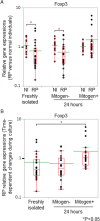
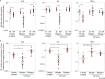
Relapsing polychondritis (RP) is an inflammatory disease of unknown causes, characterized by recurrent inflammation in cartilaginous tissues of the whole body. Recently, researchers have reported that, in mouse experiments, altered gut microbe-dependent T cell differentiation occurred in gut associated lymphoid tissues. Here, we investigated whether gut microbe alteration existed, and if so, the alteration affected peripheral T cell differentiation in patients with RP. In an analysis of gut microbiota, we found increased annotated species numbers in RP patients compared with normal individuals. In the RP gut microbiota, we observed several predominant species, namely Veillonella parvula, Bacteroides eggerthii, Bacteroides fragilis, Ruminococcus bromii, and Eubacterium dolichum, all species of which were reported to associate with propionate production in human intestine. Propionate is a short-chain fatty acid and is suggested to associate with interleukin (IL)10-producing regulatory T (Treg) cell differentiation in gut associated lymphoid tissues. IL10 gene expressions were moderately higher in freshly isolated peripheral blood mononuclear cells (PBMC) of RP patients than those of normal individuals. Six hours after the initiation of the cell culture, regardless of the presence and absence of mitogen stimulation, IL10 gene expressions were significantly lower in RP patients than those in normal individuals. It is well known that PBMC of patients with autoimmune and inflammatory diseases show hyporesponsiveness to mitogen stimulation. We suggest that, in RP patients, continuous stimulation of intestinal T cells by excessive propionate leads to the spontaneous IL10 production and a subsequent refractory period of T cells in patients with RP. The hyporesponsiveness of Treg cells upon activation may associate with inflammatory cytokine production of PBMC and subsequently relate to chondritis in RP patients.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Letko E, Zafirakis P, Baltatzis S, Voudouri A, Livir-Rallatos C, Foster CS. Relapsing polychondritis: a clinical review. Semin Arthritis Rheum. 2002; 31: 384–395. - PubMed
-
- Oka H, Yamano Y, Shimizu J, Yudoh K, Suzuki N. A large-scale survey of patients with relapsing polychondritis in Japan. Inflamm Regen. 2014; 34: 149–156.
-
- Hansson AS, Heinegård D, Piette JC, Burkhardt H, Holmdahl R. The occurrence of autoantibodies to matrilin 1 reflects a tissue-specific response to cartilage of the respiratory tract in patients with relapsing polychondritis. Arthritis Rheum. 2001; 44: 2402–2412. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

