Safety and tolerability of HIV-1 multiantigen pDNA vaccine given with IL-12 plasmid DNA via electroporation, boosted with a recombinant vesicular stomatitis virus HIV Gag vaccine in healthy volunteers in a randomized, controlled clinical trial
- PMID: 30235286
- PMCID: PMC6147413
- DOI: 10.1371/journal.pone.0202753
Safety and tolerability of HIV-1 multiantigen pDNA vaccine given with IL-12 plasmid DNA via electroporation, boosted with a recombinant vesicular stomatitis virus HIV Gag vaccine in healthy volunteers in a randomized, controlled clinical trial
Abstract
Background: The addition of plasmid cytokine adjuvants, electroporation, and live attenuated viral vectors may further optimize immune responses to DNA vaccines in heterologous prime-boost combinations. The objective of this study was to test the safety and tolerability of a novel prime-boost vaccine regimen incorporating these strategies with different doses of IL-12 plasmid DNA adjuvant.
Methods: In a phase 1 study, 88 participants received an HIV-1 multiantigen (gag/pol, env, nef/tat/vif) DNA vaccine (HIV-MAG, 3000 μg) co-administered with IL-12 plasmid DNA adjuvant at 0, 250, 1000, or 1500 μg (N = 22/group) given intramuscularly with electroporation (Ichor TriGrid™ Delivery System device) at 0, 1 and 3 months; followed by attenuated recombinant vesicular stomatitis virus, serotype Indiana, expressing HIV-1 Gag (VSV-Gag), 3.4 ⊆ 107 plaque-forming units (PFU), at 6 months; 12 others received placebo. Injections were in both deltoids at each timepoint. Participants were monitored for safety and tolerability for 15 months.
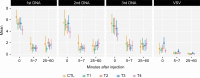
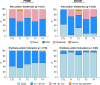
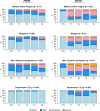
Results: The dose of IL-12 pDNA did not increase pain scores, reactogenicity, or adverse events with the co-administered DNA vaccine, or following the VSV-Gag boost. Injection site pain and reactogenicity were common with intramuscular injections with electroporation, but acceptable to most participants. VSV-Gag vaccine often caused systemic reactogenicity symptoms, including a viral syndrome (in 41%) of fever, chills, malaise/fatigue, myalgia, and headache; and decreased lymphocyte counts 1 day after vaccination.
Conclusions: HIV-MAG DNA vaccine given by intramuscular injection with electroporation was safe at all doses of IL-12 pDNA. The VSV-Gag vaccine at this dose was associated with fever and viral symptoms in some participants, but the vaccine regimens were safe and generally well-tolerated.
Trial registration: Clinical Trials.gov NCT01578889.
Conflict of interest statement
The authors have read the journal policy and the authors of this manuscript have the following competing interests: MAA is employed by the National Institute of Allergy and Infectious Diseases (NIAID), the study sponsor. MLE, SSL, NKK, GJW, HVNT, IF, MES, KWC, BSP, JHE, MJM and CMH are recipients of NIAID funding, and this publication is a result of activities funded by NIAID. MAA is not involved with the process of funding these awards, nor in their administration of scientific aspects and, in accordance with NIAID policies, is deferred from decisions regarding funding of coauthors for a requisite period. TEL, DKC, JHE, RX, and AOS are employees and stakeholders of Profectus BioSciences, Inc. DKC is the principal author/inventor on a patent for the rVSVN4CT1 vector. At the time the study was conducted, MAE was an employee and stakeholder of Profectus BioSciences, Inc. and is now an employee of Takeda Pharmaceuticals, which has no affiliation with the study. DH is an employee and stakeholder of Ichor Medical Systems, Inc. IF serves on advisory boards for Gilead Sciences and ViiV Healthcare. HVNT has received research grant funding from Merck & Co. These affiliations do not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures


References
-
- Dunachie SJ, Hill AV. Prime-boost strategies for malaria vaccine development. J Exp Biol. 2003;206(Pt 21):3771–9. . - PubMed
-
- Schneider J, Gilbert SC, Hannan CM, Degano P, Prieur E, Sheu EG, et al. Induction of CD8+ T cells using heterologous prime-boost immunisation strategies. Immunol Rev. 1999;170:29–38. - PubMed
-
- Kraynyak KA, Kutzler MA, Cisper NJ, Laddy DJ, Morrow MP, Waldmann TA, et al. Plasmid-encoded interleukin-15 receptor alpha enhances specific immune responses induced by a DNA vaccine in vivo. Hum Gene Ther. 2009;20(10):1143–56. 10.1089/hum.2009.025 ; PubMed Central PMCID: PMCPMC2829284. - DOI - PMC - PubMed
-
- Clarke DK, Hendry RM, Singh V, Rose JK, Seligman SJ, Klug B, et al. Live virus vaccines based on a vesicular stomatitis virus (VSV) backbone: Standardized template with key considerations for a risk/benefit assessment. Vaccine. 2016;34(51):6597–609. 10.1016/j.vaccine.2016.06.071 ; PubMed Central PMCID: PMCPMC5220644. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

