Relapse Rate in Survivors of Acute Autoimmune Thrombotic Thrombocytopenic Purpura Treated with or without Rituximab
- PMID: 30235478
- PMCID: PMC6202932
- DOI: 10.1055/s-0038-1668545
Relapse Rate in Survivors of Acute Autoimmune Thrombotic Thrombocytopenic Purpura Treated with or without Rituximab
Abstract
Background: Autoimmune thrombotic thrombocytopenic purpura (iTTP) is caused by autoantibody-mediated severe a disintegrin and metalloprotease with thrombospondin type 1 repeats, member 13 (ADAMTS13) deficiency leading to micro-angiopathic haemolytic anaemia (MAHA) and thrombocytopenia with organ damage. Patients survive with plasma exchange (PEX), fresh frozen plasma replacement and corticosteroid treatment. Anti-CD20 monoclonal antibody rituximab is increasingly used in patients resistant to conventional PEX or relapsing after an acute bout.
Objective: This retrospective observational study focused on the relapse rate and possible influencing factors including treatment with rituximab first introduced in 2003.
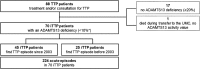
Patients and methods: Seventy patients treated between January 2003 and November 2014 were evaluated. Number, duration, clinical manifestations, laboratory data and treatment of acute episodes were documented. Diagnostic criteria of acute iTTP were thrombocytopenia, MAHA, increased lactate dehydrogenase and severe ADAMTS13 deficiency.
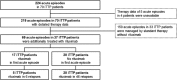
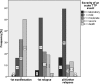
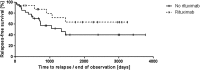
Results: Fifty-four female and 16 male patients had a total of 224 acute episodes over a median observation period of 8.3 years. The relapse rate was 2.6% per month, for women 2.4% and for men 3.5% per month. Since 2003, 17 patients with a first iTTP episode were treated with rituximab, whereas 28 were not. There was a trend towards lower relapse rates after rituximab treatment over the ensuing years. However, this was statistically not significant.
Conclusion: This analysis does not show a significant reduction of acute iTTP relapses by rituximab given during an acute bout. Initial episodes are characterized by more severe clinical signs compared with the less severe relapses. Furthermore, men suffer significantly more frequent and considerably more serious acute relapses.
Georg Thieme Verlag KG Stuttgart · New York.
Conflict of interest statement
The authors declare that they have no conflicts of interest relevant to the manuscript. I. Scharrer is a member of the Data Safety Monitoring Board in the BAX 930 study (investigating recombinant ADAMTS13 infusion in hereditary TTP). She received travel and accommodation support for participating at scientific congresses or meetings from Bayer and NovoNordisk. B. Lämmle is chairman of the Data Safety Monitoring Board in the BAX 930 study (investigating recombinant ADAMTS13 infusion in hereditary TTP). He is on the Advisory Board of Ablynx for the development of caplacizumab. He holds a patent on ADAMTS13 and received travel and accommodation support for participating at scientific congresses or meetings from Baxalta, Siemens, Alexion, Ablynx and Bayer and speaker's fees from Siemens, Bayer and Alexion.
Figures

Similar articles
-
Preemptive rituximab prevents long-term relapses in immune-mediated thrombotic thrombocytopenic purpura.Blood. 2018 Nov 15;132(20):2143-2153. doi: 10.1182/blood-2018-04-840090. Epub 2018 Sep 10. Blood. 2018. PMID: 30201758
-
Child-onset and adolescent-onset acquired thrombotic thrombocytopenic purpura with severe ADAMTS13 deficiency: a cohort study of the French national registry for thrombotic microangiopathy.Lancet Haematol. 2016 Nov;3(11):e537-e546. doi: 10.1016/S2352-3026(16)30125-9. Epub 2016 Oct 3. Lancet Haematol. 2016. PMID: 27720178
-
Rituximab as pre-emptive treatment in patients with thrombotic thrombocytopenic purpura and evidence of anti-ADAMTS13 autoantibodies.Thromb Haemost. 2009 Feb;101(2):233-8. Thromb Haemost. 2009. PMID: 19190804
-
Thrombotic thrombocytopenic purpura.Blood. 2017 May 25;129(21):2836-2846. doi: 10.1182/blood-2016-10-709857. Epub 2017 Apr 17. Blood. 2017. PMID: 28416507 Review.
-
Long-term risk of relapse in immune-mediated thrombotic thrombocytopenic purpura and the role of anti-CD20 therapy.Blood. 2023 Jan 19;141(3):285-294. doi: 10.1182/blood.2022017023. Blood. 2023. PMID: 36322971 Review.
Cited by
-
A novel von Willebrand factor multimer ratio as marker of disease activity in thrombotic thrombocytopenic purpura.Blood Adv. 2023 Sep 12;7(17):5091-5102. doi: 10.1182/bloodadvances.2023010028. Blood Adv. 2023. PMID: 37399489 Free PMC article.
-
Laboratory surveillance of immune-mediated thrombotic thrombocytopenic purpura.Hematology Am Soc Hematol Educ Program. 2020 Dec 4;2020(1):82-84. doi: 10.1182/hematology.2020000164. Hematology Am Soc Hematol Educ Program. 2020. PMID: 33275690 Free PMC article. Review. No abstract available.
-
Thrombotic Thrombocytopenic Purpura: Pathophysiology, Diagnosis, and Management.J Clin Med. 2021 Feb 2;10(3):536. doi: 10.3390/jcm10030536. J Clin Med. 2021. PMID: 33540569 Free PMC article. Review.
-
Cost effectiveness of caplacizumab in acquired thrombotic thrombocytopenic purpura.Blood. 2021 Feb 18;137(7):969-976. doi: 10.1182/blood.2020006052. Blood. 2021. PMID: 33280030 Free PMC article.
-
The efficacy and safety of caplacizumab in Japanese patients with immune-mediated thrombotic thrombocytopenic purpura: an open-label phase 2/3 study.Int J Hematol. 2023 Mar;117(3):366-377. doi: 10.1007/s12185-022-03495-6. Epub 2022 Nov 24. Int J Hematol. 2023. PMID: 36427162 Free PMC article. Clinical Trial.
References
-
- Furlan M, Robles R, Lämmle B. Partial purification and characterization of a protease from human plasma cleaving von Willebrand factor to fragments produced by in vivo proteolysis. Blood. 1996;87(10):4223–4234. - PubMed
-
- Coppo P, Veyradier A. Thrombotic microangiopathies: towards a pathophysiology-based classification. Cardiovasc Hematol Disord Drug Targets. 2009;9(01):36–50. - PubMed
-
- Scully M, Hunt B J, Benjamin S et al.Guidelines on the diagnosis and management of thrombotic thrombocytopenic purpura and other thrombotic microangiopathies. Br J Haematol. 2012;158(03):323–335. - PubMed
-
- Kremer Hovinga J A, Vesely S K, Terrell D R, Lämmle B, George J N. Survival and relapse in patients with thrombotic thrombocytopenic purpura. Blood. 2010;115(08):1500–1511. - PubMed
-
- George J N. How I treat patients with thrombotic thrombocytopenic purpura: 2010. Blood. 2010;116(20):4060–4069. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

