Patients with chronic hepatitis C receiving sofosbuvir and ribavirin-based treatment, with or without interferon in Zhejiang, China: An observational study
- PMID: 30235711
- PMCID: PMC6160050
- DOI: 10.1097/MD.0000000000012403
Patients with chronic hepatitis C receiving sofosbuvir and ribavirin-based treatment, with or without interferon in Zhejiang, China: An observational study
Abstract
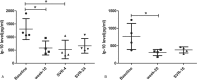
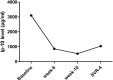
Hepatitis C virus (HCV) is one of the most important virus as the cause of liver disease in China. The aim of the present study was to explore whether sofosbuvir and ribavirin-based treatment can cure patients with chronic hepatitis C in eastern China. We examined a cohort of HCV-monoinfected patients and 9 patients agreed to participate in our treatment and research. The patients were diagnosed with chronic hepatitis C with or without cirrhosis. Nine patients including 4 female and 5 male met the requirements for selection and were willing to participate in this experiment. Sofosbuvir and ribavirin-based treatment with or without interferon was given to the patients. Viral loads, cytokines, and chemokines were recorded during treatment and after treatment. After 2 weeks of sofosbuvir and ribavirin-based treatment, the viral load of patients decreased to limits of detection. Eight patients were cured. Patients had rapid virological response (RVR) with undetectable viral load at week 4 and sustained virological response (SVR). The interferon-inducible protein-10 (IP-10) decreased after the treatment. However, the patient with cirrhosis failed, as the virus reappeared during SVR4. At the same time, the IP-10 dramatically increased as the relapse of the HCV virus. In summary, the IP-10 has the potential to be the biomarker for the prognostic of HCV.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures
Similar articles
-
Treatment of chronic genotype-3 hepatitis C virus infection using direct-acting antiviral agents: An Indian experience.Indian J Gastroenterol. 2017 May;36(3):227-234. doi: 10.1007/s12664-017-0763-3. Epub 2017 Jun 28. Indian J Gastroenterol. 2017. PMID: 28656492
-
Safety and Effectiveness of Ledipasvir and Sofosbuvir, With or Without Ribavirin, in Treatment-Experienced Patients With Genotype 1 Hepatitis C Virus Infection and Cirrhosis.Clin Gastroenterol Hepatol. 2018 Nov;16(11):1811-1819.e4. doi: 10.1016/j.cgh.2017.12.037. Epub 2018 Jan 3. Clin Gastroenterol Hepatol. 2018. PMID: 29306043 Free PMC article.
-
Sofosbuvir and ribavirin for genotype 2 HCV infected patients with cirrhosis: A real life experience.J Hepatol. 2017 Apr;66(4):711-717. doi: 10.1016/j.jhep.2016.12.002. Epub 2016 Dec 10. J Hepatol. 2017. PMID: 27965158
-
Results of sofosbuvir-based combination therapy for chronic hepatitis C cohort of Indian patients in real-life clinical practice.J Gastroenterol Hepatol. 2017 Apr;32(4):894-900. doi: 10.1111/jgh.13628. J Gastroenterol Hepatol. 2017. PMID: 27787910 Clinical Trial.
-
Sofosbuvir, ribavirin and pegylated interferon for a daclatasvir-resistent genotype 3 hepatitis C virus: case report and review.Rev Inst Med Trop Sao Paulo. 2019 Feb 14;61:e12. doi: 10.1590/S1678-9946201961012. Rev Inst Med Trop Sao Paulo. 2019. PMID: 30785566 Free PMC article. Review.
Cited by
-
Association of cytokine gene polymorphisms with chronic hepatitis C virus genotype 1b infection in Chinese Han population: An observational study.Medicine (Baltimore). 2020 Sep 18;99(38):e22362. doi: 10.1097/MD.0000000000022362. Medicine (Baltimore). 2020. PMID: 32957410 Free PMC article.
-
Prediction of response to sofosbuvir-based therapy using serum interleukin-12 and single nucleotide polymorphism of the interleukin 28B gene as predictive factors in HCV positive genotype-4 patients.Medicine (Baltimore). 2023 Jul 14;102(28):e34125. doi: 10.1097/MD.0000000000034125. Medicine (Baltimore). 2023. PMID: 37443472 Free PMC article.
References
-
- Mohd Hanafiah K, Groeger J, Flaxman AD, et al. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology 2013;57:1333–42. - PubMed
-
- Polaris Observatory HCVC. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol 2017;2:161–76. - PubMed
-
- Lim SG, Aghemo A, Chen PJ, et al. Management of hepatitis C virus infection in the Asia-Pacific region: an update. Lancet Gastroenterol Hepatol 2017;2:52–62. - PubMed
-
- Razavi H, Robbins S, Zeuzem S, et al. Hepatitis C virus prevalence and level of intervention required to achieve the WHO targets for elimination in the European Union by 2030: a modelling study. Lancet Gastroenterol Hepatol 2017;2:325–36. - PubMed
-
- Chen YS, Li L, Cui FQ, et al. A sero-epidemiological study on hepatitis C in China. Chin J Epidemiol 2011;32:888–91. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

