Hepatic toxicity following actinomycin D chemotherapy in treatment of familial gestational trophoblastic neoplasia: A case report
- PMID: 30235719
- PMCID: PMC6160083
- DOI: 10.1097/MD.0000000000012424
Hepatic toxicity following actinomycin D chemotherapy in treatment of familial gestational trophoblastic neoplasia: A case report
Abstract
Rationale: Familial hydatidiform mole is extremely rare while familial gestational trophoblastic neoplasia (GTN) has never been reported. Inspired by 2 biological sisters with postmolar GTN and liver toxicity, we reviewed susceptible maternal-effect genes and explored the role of possible drug transporter genes in the development of GTN.
Patient concerns: We reported one Chinese family where the two sisters developed postmolar GTN while experiencing fast remission and significant hepatic toxicity from actinomycin D chemotherapy.
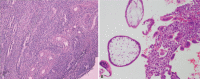
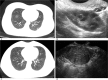
Diagnoses: The index pregnancy was diagnosed with curettage. The following GTN was confirmed when there was a rise in beta-hCG for three consecutive weekly measurements over at least a period of 2 weeks. Computed tomography was used to identify lung metastasis. The elder sister was diagnosed with gestational trophoblastic neoplasia (III: 2) while the younger sister was diagnosed as III: 3 according to WHO scoring system.
Interventions: Patients were treated with actinomycin D of 10 μg/kg intravenously for 5 days every 2 weeks. When hepatic toxicity was indicated, polyene phosphatidyl choline and magnesium isoglycyrrhizinate were prescribed.
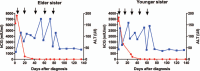
Outcomes: Both patients responded extremely well to the 5-day actinomycin D regimen. Beta-hCG remained less than 2 mIU/ml after 5 cycles while computed tomography scan showed downsized pulmonary nodules. Both experienced significant rise in ALT and AST levels that could be ameliorated with corresponding medication. Monthly followed-up showed negative beta-hCG levels and normal liver enzyme levels.
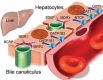
Lessons: We speculated that the known or unknown NLRP7 and KHDC3L mutations might be correlated with drug disposition in liver while liver drug transporters such as P-glycoprotein family that are also expressed in trophoblasts might be correlated to GTN susceptibility. Future genomic profiles of large samples alike using next generation sequencing are needed to confirm our hypothesis and discover yet unknown genes.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures
Similar articles
-
Effectiveness and toxicity of second-line actinomycin D in patients with methotrexate-resistant postmolar low-risk gestational trophoblastic neoplasia.Gynecol Oncol. 2020 May;157(2):372-378. doi: 10.1016/j.ygyno.2020.02.001. Epub 2020 Feb 7. Gynecol Oncol. 2020. PMID: 32037196
-
Pulse methotrexate versus pulse actinomycin D in the treatment of low-risk gestational trophoblastic neoplasia.Int J Gynaecol Obstet. 2008 Oct;103(1):33-7. doi: 10.1016/j.ijgo.2008.05.013. Epub 2008 Jul 16. Int J Gynaecol Obstet. 2008. PMID: 18632105 Clinical Trial.
-
Prevention of postmolar gestational trophoblastic neoplasia using prophylactic single bolus dose of actinomycin D in high-risk hydatidiform mole: a simple, effective, secure and low-cost approach without adverse effects on compliance to general follow-up or subsequent treatment.Gynecol Oncol. 2009 Aug;114(2):299-305. doi: 10.1016/j.ygyno.2009.04.006. Epub 2009 May 8. Gynecol Oncol. 2009. PMID: 19427681
-
Does a human chorionic gonadotropin level of over 20,000 IU/L four weeks after uterine evacuation for complete hydatidiform mole constitute an indication for chemotherapy for gestational trophoblastic neoplasia?Eur J Obstet Gynecol Reprod Biol. 2018 Apr;223:50-55. doi: 10.1016/j.ejogrb.2018.02.001. Epub 2018 Feb 15. Eur J Obstet Gynecol Reprod Biol. 2018. PMID: 29477553 Review.
-
Multiple metastatic gestational trophoblastic disease after a twin pregnancy with complete hydatidiform mole and coexisting fetus, following assisted reproductive technology: Case report and literature review.Taiwan J Obstet Gynecol. 2018 Aug;57(4):588-593. doi: 10.1016/j.tjog.2018.06.020. Taiwan J Obstet Gynecol. 2018. PMID: 30122584 Review.
Cited by
-
High-Risk Gestational Trophoblastic Neoplasia from a Homozygous NLRP7 Mutation.Gynecol Oncol Rep. 2021 Jun 6;37:100803. doi: 10.1016/j.gore.2021.100803. eCollection 2021 Aug. Gynecol Oncol Rep. 2021. PMID: 34189227 Free PMC article.
-
Effectiveness and Economic Evaluation of Polyene Phosphatidyl Choline in Patients With Liver Diseases Based on Real-World Research.Front Pharmacol. 2022 Mar 7;13:806787. doi: 10.3389/fphar.2022.806787. eCollection 2022. Front Pharmacol. 2022. PMID: 35330831 Free PMC article.
References
-
- Ngan HY, Seckl MJ, Berkowitz RS, et al. Update on the diagnosis and management of vgestational trophoblastic disease. Int J Gynaecol Obstet 2015;131(suppl 2):S123–6. - PubMed
-
- Seckl MJ, Sebire NJ, Berkowitz RS. Gestational trophoblastic disease. Lancet 2010;376:717–29. - PubMed
-
- Coyle C, Short D, Jackson L, et al. What is the optimal duration of human chorionic gonadotrophin surveillance following evacuation of a molar pregnancy? A retrospective analysis on over 20,000 consecutive patients. Gynecol Oncol 2018;148:254–7. - PubMed
-
- Jiang F, Wan XR, Xu T, et al. Evaluation and suggestions for improving the FIGO 2000 staging criteria for gestational trophoblastic neoplasia: a ten-year review of 1420 patients. Gynecol Oncol 2018;18:30271–3. - PubMed
-
- Melamed A, Gockley AA, Joseph NT, et al. Effect of race/ethnicity on risk of complete and partial molar pregnancy after adjustment for age. Gynecol Oncol 2016;143:73–6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

