Clinical and radiologic analysis of on-demand use of etanercept for disease flares in patients with rheumatoid arthritis for 2 years: The RESUME study: A case-control study
- PMID: 30235736
- PMCID: PMC6160256
- DOI: 10.1097/MD.0000000000012462
Clinical and radiologic analysis of on-demand use of etanercept for disease flares in patients with rheumatoid arthritis for 2 years: The RESUME study: A case-control study
Abstract
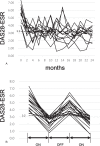
To reduce costs of biological disease-modifying antirheumatic drugs (bDMARDs), we evaluated the efficacy of repeated etanercept (ETN) discontinuation and restarting in rheumatoid arthritis (RA) patients in a case-control study.Thirty-one bDMARD-naive RA patients with moderate to high disease activity received ETN until low disease activity (LDA) was achieved, after which ETN was discontinued. Upon flaring, ETN was readministered with observation every 2 months for 2 years, and radiographically evaluated in comparison with a historical control group treated continuously with ETN. Statistical methods including Fisher exact test, analysis of variance (ANOVA), Kruskal-Wallis test, multiple regression analysis, and Student t test were conducted as appropriate.Thirteen patients with inadequate response to ETN were withdrawn from the study, and 5 had no flare-up after ETN discontinuation. In the remaining 13 patients, ETN was used on-demand to maintain LDA. Multivariate analysis revealed that MTX was significantly correlated with ETN. All 13 patients achieved LDA at final follow-up. Although joint damage progressed in patients using ETN on-demand, structural damage progression in the on-demand group was not significantly different from that in controls.On-demand use of ETN for flaring reduced disease activity but not structural damage in 50% of patients (though not significantly). However, inhibition of joint damage was achieved in 50% of patients after 2 years, supporting on-demand use of ETN as a treatment option for patients with RA who cannot afford bDMARD or targeted synthetic DMARD therapy.
Conflict of interest statement
Conflicts of interest: K.I. has received speakers fee and/or research grant from Chugai Pharmaceutical Co., Ltd, Mitsubishi Tanabe Pharma Co., Astellas Pharma, Inc., Abbvie GK, Eisai Co., Ltd, Takeda Pharmaceutical Co., Ltd, Daiichi Sankyo Co., Ltd, Ono Pharmaceutical Co., Ltd, Janssen Pharmaceutical K.K., Pfizer Japan, Inc., Eisai Co., Ltd, Sanofi K.K., Qol Co., Ltd.
All other authors have declared no conflicts of interest.
Figures
Similar articles
-
Treatment Patterns and Costs in Biologic DMARD-Naive Patients with Rheumatoid Arthritis Initiating Etanercept or Adalimumab with or Without Methotrexate.J Manag Care Spec Pharm. 2020 Mar;26(3):285-294. doi: 10.18553/jmcp.2020.26.3.285. J Manag Care Spec Pharm. 2020. PMID: 32105179 Free PMC article.
-
Full dose, reduced dose or discontinuation of etanercept in rheumatoid arthritis.Ann Rheum Dis. 2016 Jan;75(1):52-8. doi: 10.1136/annrheumdis-2014-205726. Epub 2015 Apr 14. Ann Rheum Dis. 2016. PMID: 25873634 Free PMC article. Clinical Trial.
-
Discontinuation of disease-modifying anti-rheumatic drugs and clinical outcomes in the Rheumatoid Arthritis DMARD Intervention and Utilisation Study 2 (RADIUS 2).Clin Exp Rheumatol. 2015 May-Jun;33(3):297-301. Epub 2015 Feb 18. Clin Exp Rheumatol. 2015. PMID: 25738333
-
Pharmacokinetics, efficacy and safety profiles of etanercept monotherapy in Japanese patients with rheumatoid arthritis: review of seven clinical trials.Mod Rheumatol. 2015 Mar;25(2):173-86. doi: 10.3109/14397595.2014.914014. Epub 2014 May 20. Mod Rheumatol. 2015. PMID: 24842477 Free PMC article. Review.
-
Dosing down and then discontinuing biologic therapy in rheumatoid arthritis: a review of the literature.Int J Rheum Dis. 2018 Feb;21(2):362-372. doi: 10.1111/1756-185X.13238. Epub 2017 Dec 4. Int J Rheum Dis. 2018. PMID: 29205904 Review.
Cited by
-
Prevalence and predictors of sustained remission/low disease activity after discontinuation of induction or maintenance treatment with tumor necrosis factor inhibitors in rheumatoid arthritis: a systematic and scoping review.Arthritis Res Ther. 2023 Nov 20;25(1):222. doi: 10.1186/s13075-023-03199-0. Arthritis Res Ther. 2023. PMID: 37986101 Free PMC article.
-
Use of external control arms in immune-mediated inflammatory diseases: a systematic review.BMJ Open. 2023 Dec 9;13(12):e076677. doi: 10.1136/bmjopen-2023-076677. BMJ Open. 2023. PMID: 38070932 Free PMC article.
References
-
- Singh JA, Saag KG, Bridges SL, Jr, et al. 2015 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Care Res (Hoboken) 2016;68:1–25. - PubMed
-
- Smolen JS, Landewe R, Bijlsma J, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis 2017;76:960–77. - PubMed
-
- Michaud K, Messer J, Choi HK, et al. Direct medical costs and their predictors in patients with rheumatoid arthritis: a three-year study of 7,527 patients. Arthritis Rheum 2003;48:2750–62. - PubMed
-
- Sokka T, Haugeberg G, Asikainen J, et al. Similar clinical outcomes in rheumatoid arthritis with more versus less expensive treatment strategies. Observational data from two rheumatology clinics. Clin Exp Rheumatol 2013;31:409–14. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

