Effectiveness of binocularity-stimulating treatment in children with residual amblyopia following occlusion
- PMID: 30236086
- PMCID: PMC6149203
- DOI: 10.1186/s12886-018-0922-z
Effectiveness of binocularity-stimulating treatment in children with residual amblyopia following occlusion
Abstract
Background: To evaluate the effectiveness of binocularity-stimulating treatment in children with residual amblyopia following occlusion therapy for more than 6 months.
Methods: Of patients with amblyopia caused by anisometropia and/or strabismus, patients with residual amblyopia following more than 6 months of occlusion therapy were included. Subjects underwent one of the following types of binocularity-stimulating therapy: Bangerter foil (BF), head-mounted display (HMD) game, or BF/HMD combination (BF + HMD). Factors including age, sex, types of amblyopia, visual acuity, and duration of treatment were investigated. Baseline and final (after at least 2 months of treatment) visual acuity were also compared.
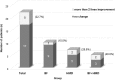
Results: Twenty-two patients with a mean age of 8.7 ± 1.3 years were included. Seven patients had anisometropic amblyopia, 8 patients had strabismic amblyopia, and 7 patients had combined amblyopia. After 4.4 ± 1.8 months of treatment, logarithm of the minimum angle of resolution (logMAR) visual acuity in the amblyopic eye improved from 0.22 ± 0.20 to 0.18 ± 0.15. Five of 22 patients (22.7%) gained more than 0.2 logMAR, including 1 of 10 patients (10.0%) in the BF group, 2 of 7 patients (28.6%) in the HMD group, and 2 of 5 patients (40.0%) in the BF + HMD group. No significant differences in clinical characteristics were identified among the three groups.
Conclusions: Binocularity-stimulating therapy is somewhat beneficial in children with residual amblyopia and might be attempted when children no longer benefit from sufficient long-term period of occlusion therapy.
Keywords: Amblyopia; Binocular treatment; Binocularity-stimulating treatment; Residual amblyopia.
Conflict of interest statement
Ethics approval and consent to participate
This study received ethical approval from the Institutional Review Board of the Seoul National University Hospital. The Ethics Committee study protocol number was 1706–205-866 and the IRB granted a waiver of consent for this retrospective chart review study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
-
- Levartovsky S, Gottesman N, Shimshoni M, Oliver M. Factors affecting long-term results of successfully treated amblyopia: age at beginning of treatment and age at cessation of monitoring. J Pediatr Ophthalmol Strabismus. 1992;29(4):219–223. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

