Short and long-term effects of pulmonary rehabilitation in interstitial lung diseases: a randomised controlled trial
- PMID: 30236104
- PMCID: PMC6149060
- DOI: 10.1186/s12931-018-0884-y
Short and long-term effects of pulmonary rehabilitation in interstitial lung diseases: a randomised controlled trial
Abstract
Background: Few data are available on the long-term effect of pulmonary rehabilitation (PR) and on long PR programs in interstitial lung diseases (ILD). We aimed to evaluate the effects of PR on exercise capacity (6-Minute Walking Distance, 6MWD; Peak Work Rate, Wmax), quality of life (St George's Respiratory Questionnaire, SGRQ), quadriceps force (QF) and objectively measured physical activity in ILD after the 6-month PR-program and after 1 year.
Methods: 60 patients (64 ± 11 years; 62% males; 23% with IPF) were randomly assigned to receive a 6 month-PR program or usual medical care.
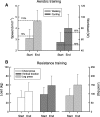
Results: Exercise capacity, quality of life and muscle force increased significantly after the program as compared to control (mean,95%CI[ll to ul]; 6MWD + 72,[36 to 108] m; Wmax 19, [8 to 29]%pred; SGRQ - 12,[- 19 to - 6] points; QF 10, [1 to 18] %pred). The gain was sustained after 1 year (6MWD 73,[28 to 118] m; Wmax 23, [10 to 35]%pred; SGRQ - 11,[- 18 to - 4] points; QF 9.5, [1 to 18] %pred). Physical activity did not change.
Conclusions: PR improves exercise tolerance, health status and muscle force in ILD. The benefits are maintained at 1-year follow-up. The intervention did not change physical activity.
Trial registration: Clinicaltrials.gov NCT00882817 .
Conflict of interest statement
Ethics approval and consent to participate
The local ethics committee of the university hospital of Leuven approved the study on 22-01-2009 with the committee’s reference number B32220095560.
Consent for publication
Not applicable.
Competing interests
SPB, VB, HD and TT have nothing to disclose. WW reports grants from Roche and Boehringer Ingelheim, outside the submitted work. WJ reports grants from Chiesi, Astra Zeneca, Boehringer Ingelheim, GSK, Novartis, outside the submitted work.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


References
-
- Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, Colby TV, Cordier JF, Flaherty KR, Lasky JA, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788–824. doi: 10.1164/rccm.2009-040GL. - DOI - PMC - PubMed
-
- Travis WD, Costabel U, Hansell DM, King TE, Jr, Lynch DA, Nicholson AG, Ryerson CJ, Ryu JH, Selman M, Wells AU, et al. An official American Thoracic Society/European Respiratory Society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2013;188:733–748. doi: 10.1164/rccm.201308-1483ST. - DOI - PMC - PubMed
-
- Raghu G, Rochwerg B, Zhang Y, Garcia CA, Azuma A, Behr J, Brozek JL, Collard HR, Cunningham W, Homma S, et al. An official ATS/ERS/JRS/ALAT clinical practice guideline: treatment of idiopathic pulmonary fibrosis. An update of the 2011 clinical practice guideline. Am J Respir Crit Care Med. 2015;192:e3–19. doi: 10.1164/rccm.201506-1063ST. - DOI - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

